Impact of G-Quadruplexes on the Regulation of Genome Integrity, DNA Damage and Repair
- PMID: 34572497
- PMCID: PMC8472537
- DOI: 10.3390/biom11091284
Impact of G-Quadruplexes on the Regulation of Genome Integrity, DNA Damage and Repair
Abstract
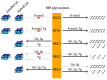
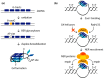
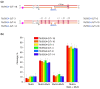
DNA G-quadruplexes (G4s) are known to be an integral part of the complex regulatory systems in both normal and pathological cells. At the same time, the ability of G4s to impede DNA replication plays a critical role in genome integrity. This review summarizes the results of recent studies of G4-mediated genomic and epigenomic instability, together with associated DNA damage and repair processes. Although the underlying mechanisms remain to be elucidated, it is known that, among the proteins that recognize G4 structures, many are linked to DNA repair. We analyzed the possible role of G4s in promoting double-strand DNA breaks, one of the most deleterious DNA lesions, and their repair via error-prone mechanisms. The patterns of G4 damage, with a focus on the introduction of oxidative guanine lesions, as well as their removal from G4 structures by canonical repair pathways, were also discussed together with the effects of G4s on the repair machinery. According to recent findings, there must be a delicate balance between G4-induced genome instability and G4-promoted repair processes. A broad overview of the factors that modulate the stability of G4 structures in vitro and in vivo is also provided here.
Keywords: DNA damage; DNA repair; G-quadruplex; G-quadruplex-binding proteins; genomic instability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The repair of G-quadruplex-induced DNA damage.Exp Cell Res. 2014 Nov 15;329(1):178-83. doi: 10.1016/j.yexcr.2014.08.038. Epub 2014 Sep 1. Exp Cell Res. 2014. PMID: 25193076 Review.
-
The Relevance of G-Quadruplexes for DNA Repair.Int J Mol Sci. 2021 Nov 22;22(22):12599. doi: 10.3390/ijms222212599. Int J Mol Sci. 2021. PMID: 34830478 Free PMC article. Review.
-
Stable G-quadruplex DNA structures promote replication-dependent genome instability.J Biol Chem. 2022 Jun;298(6):101947. doi: 10.1016/j.jbc.2022.101947. Epub 2022 Apr 18. J Biol Chem. 2022. PMID: 35447109 Free PMC article.
-
DNA repair protein RAD52 is required for protecting G-quadruplexes in mammalian cells.J Biol Chem. 2023 Jan;299(1):102770. doi: 10.1016/j.jbc.2022.102770. Epub 2022 Dec 5. J Biol Chem. 2023. PMID: 36470428 Free PMC article.
-
G-quadruplex resolution: From molecular mechanisms to physiological relevance.DNA Repair (Amst). 2023 Oct;130:103552. doi: 10.1016/j.dnarep.2023.103552. Epub 2023 Aug 3. DNA Repair (Amst). 2023. PMID: 37572578 Review.
Cited by
-
Effects of G-Quadruplex-Binding Plant Secondary Metabolites on c-MYC Expression.Int J Mol Sci. 2022 Aug 16;23(16):9209. doi: 10.3390/ijms23169209. Int J Mol Sci. 2022. PMID: 36012470 Free PMC article.
-
Nanoscale Interaction of Endonuclease APE1 with DNA.Int J Mol Sci. 2024 May 9;25(10):5145. doi: 10.3390/ijms25105145. Int J Mol Sci. 2024. PMID: 38791183 Free PMC article.
-
DNA fragility at topologically associated domain boundaries is promoted by alternative DNA secondary structure and topoisomerase II activity.Nucleic Acids Res. 2024 Apr 24;52(7):3837-3855. doi: 10.1093/nar/gkae164. Nucleic Acids Res. 2024. PMID: 38452213 Free PMC article.
-
Human TLS DNA polymerase: saviors or threats under replication stress?Mol Cell Biochem. 2025 May 23. doi: 10.1007/s11010-025-05291-2. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40407973 Review.
-
DNA Base Excision Repair Intermediates Influence Duplex-Quadruplex Equilibrium.Molecules. 2023 Jan 18;28(3):970. doi: 10.3390/molecules28030970. Molecules. 2023. PMID: 36770637 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

