An Arsenite Relay between PSMD14 and AIRAP Enables Revival of Proteasomal DUB Activity
- PMID: 34572530
- PMCID: PMC8465394
- DOI: 10.3390/biom11091317
An Arsenite Relay between PSMD14 and AIRAP Enables Revival of Proteasomal DUB Activity
Abstract
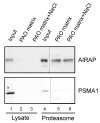
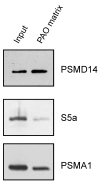
Maintaining 26S proteasome activity under diverse physiological conditions is a fundamental requirement in order to maintain cellular proteostasis. Several quantitative and qualitative mechanisms have evolved to ensure that ubiquitin-proteasome system (UPS) substrates do not accumulate and lead to promiscuous protein-protein interactions that, in turn, lead to cellular malfunction. In this report, we demonstrate that Arsenite Inducible Regulatory Particle-Associate Protein (AIRAP), previously reported as a proteasomal adaptor required for maintaining proteasomal flux during arsenite exposure, can directly bind arsenite molecules. We further show that arsenite inhibits Psmd14/Rpn11 metalloprotease deubiquitination activity by substituting zinc binding to the MPN/JAMM domain. The proteasomal adaptor AIRAP is able to directly relieve PSMD14/Rpn11 inhibition. A possible metal relay between arsenylated PSMD14/Rpn11 and AIRAP may serve as a cellular mechanism that senses proteasomal inhibition to restore Psmd14/Rpn11 activity.
Keywords: AIRAP; PSMD14/RPN11; arsenite; proteasome; protein misfolding; proteostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

