Tailored Functionalization of Natural Phenols to Improve Biological Activity
- PMID: 34572538
- PMCID: PMC8467377
- DOI: 10.3390/biom11091325
Tailored Functionalization of Natural Phenols to Improve Biological Activity
Abstract
Phenols are widespread in nature, being the major components of several plants and essential oils. Natural phenols' anti-microbial, anti-bacterial, anti-oxidant, pharmacological and nutritional properties are, nowadays, well established. Hence, given their peculiar biological role, numerous studies are currently ongoing to overcome their limitations, as well as to enhance their activity. In this review, the functionalization of selected natural phenols is critically examined, mainly highlighting their improved bioactivity after the proper chemical transformations. In particular, functionalization of the most abundant naturally occurring monophenols, diphenols, lipidic phenols, phenolic acids, polyphenols and curcumin derivatives is explored.
Keywords: carvacrol; curcumin; eugenol; hispolon; hydroxytyrosol; lipidic phenols; phenolic acids; polyphenols; resveratrol; thymol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

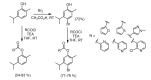

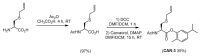
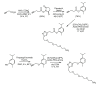

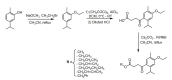
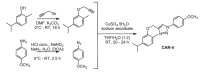









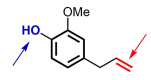
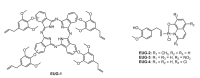

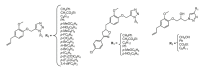

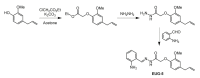
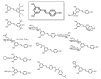



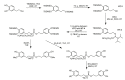
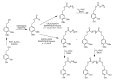
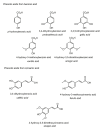


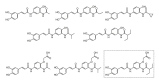




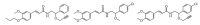

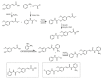
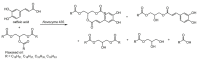
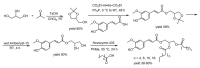

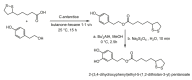


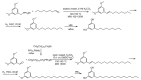
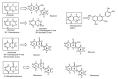

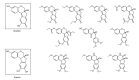






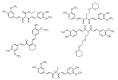


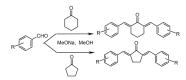





References
-
- Metsämuuronen S., Sirén H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019;18:623–664. doi: 10.1007/s11101-019-09630-2. - DOI
-
- Muhammad D.R.A., Tuenter E., Patria G.D., Foubert K., Pieters L., Dewettinck K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021;340:127983. doi: 10.1016/j.foodchem.2020.127983. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

