Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity
- PMID: 34572571
- PMCID: PMC8466754
- DOI: 10.3390/biom11091357
Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity
Abstract
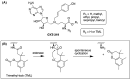
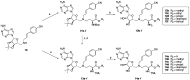
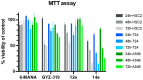
A recently discovered bisubstrate inhibitor of Nicotinamide N-methyltransferase (NNMT) was found to be highly potent in biochemical assays with a single digit nanomolar IC50 value but lacking in cellular activity. We, here, report a prodrug strategy designed to translate the observed potent biochemical inhibitory activity of this inhibitor into strong cellular activity. This prodrug strategy relies on the temporary protection of the amine and carboxylic acid moieties of the highly polar amino acid side chain present in the bisubstrate inhibitor. The modification of the carboxylic acid into a range of esters in the absence or presence of a trimethyl-lock (TML) amine protecting group yielded a range of candidate prodrugs. Based on the stability in an aqueous buffer, and the confirmed esterase-dependent conversion to the parent compound, the isopropyl ester was selected as the preferred acid prodrug. The isopropyl ester and isopropyl ester-TML prodrugs exhibit improved cell permeability, which also translates to significantly enhanced cellular activity as established using assays designed to measure the enzymatic activity of NNMT in live cells.
Keywords: NNMT; cell permeability; cellular activity; esterase; inhibition; prodrug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

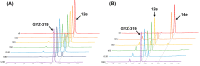
References
-
- Chlopicki S., Swies J., Mogielnicki A., Buczko W., Bartus M., Lomnicka M., Adamus J., Gebicki J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br. J. Pharmacol. 2007;152:230–239. doi: 10.1038/sj.bjp.0707383. - DOI - PMC - PubMed
-
- Mateuszuk L., Jasztal A., Maslak E., Gasior-Glogowska M., Baranska M., Sitek B., Kostogrys R., Zakrzewska A., Kij A., Walczak M., et al. Antiatherosclerotic effects of 1-methylnicotinamide in apolipoprotein e/low-density lipoprotein receptor-deficient mice: A comparison with nicotinic acid. J. Pharmacol. Exp. Ther. 2016;356:514–524. doi: 10.1124/jpet.115.228643. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

