Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension
- PMID: 34572602
- PMCID: PMC8470538
- DOI: 10.3390/biom11091389
Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension
Abstract
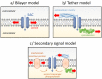
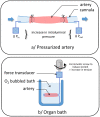
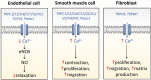
A variety of cell types in pulmonary arteries (endothelial cells, fibroblasts, and smooth muscle cells) are continuously exposed to mechanical stimulations such as shear stress and pulsatile blood pressure, which are altered under conditions of pulmonary hypertension (PH). Most functions of such vascular cells (e.g., contraction, migration, proliferation, production of extracellular matrix proteins, etc.) depend on a key event, i.e., the increase in intracellular calcium concentration ([Ca2+]i) which results from an influx of extracellular Ca2+ and/or a release of intracellular stored Ca2+. Calcium entry from the extracellular space is a major step in the elevation of [Ca2+]i, involving a variety of plasmalemmal Ca2+ channels including the superfamily of stretch-activated channels (SAC). A common characteristic of SAC is that their gating depends on membrane stretch. In general, SAC are non-selective Ca2+-permeable cation channels, including proteins of the TRP (Transient Receptor Potential) and Piezo channel superfamily. As membrane mechano-transducers, SAC convert physical forces into biological signals and hence into a cell response. Consequently, SAC play a major role in pulmonary arterial calcium homeostasis and, thus, appear as potential novel drug targets for a better management of PH.
Keywords: Piezo channel; TRP channel; calcium; endothelial cell; fibroblast; mechanosensitive channel; pulmonary arterial smooth muscle cell; pulmonary artery; pulmonary hypertension; vascular cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liu F., Haeger C.M., Dieffenbach P.B., Sicard D., Chrobak I., Coronata A.M., Suarez Velandia M.M., Vitali S., Colas R.A., Norris P.C., et al. Distal Vessel Stiffening Is an Early and Pivotal Mechanobiological Regulator of Vascular Remodeling and Pulmonary Hypertension. JCI Insight. 2016;1:e86987. doi: 10.1172/jci.insight.86987. - DOI - PMC - PubMed
-
- Galie N., Humbert M., Vachiery J.L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur. Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

