The HMGB Protein Kl Ixr1, a DNA Binding Regulator of Kluyveromyces lactis Gene Expression Involved in Oxidative Metabolism, Growth, and dNTP Synthesis
- PMID: 34572607
- PMCID: PMC8465852
- DOI: 10.3390/biom11091392
The HMGB Protein Kl Ixr1, a DNA Binding Regulator of Kluyveromyces lactis Gene Expression Involved in Oxidative Metabolism, Growth, and dNTP Synthesis
Abstract
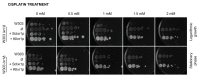
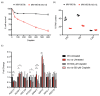
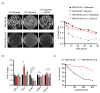
In the traditional fermentative model yeast Saccharomyces cerevisiae, ScIxr1 is an HMGB (High Mobility Group box B) protein that has been considered as an important regulator of gene transcription in response to external changes like oxygen, carbon source, or nutrient availability. Kluyveromyces lactis is also a useful eukaryotic model, more similar to many human cells due to its respiratory metabolism. We cloned and functionally characterized by different methodologies KlIXR1, which encodes a protein with only 34.4% amino acid sequence similarity to ScIxr1. Our data indicate that both proteins share common functions, including their involvement in the response to hypoxia or oxidative stress induced by hydrogen peroxide or metal treatments, as well as in the control of key regulators for maintenance of the dNTP (deoxyribonucleotide triphosphate) pool and ribosome synthesis. KlIxr1 is able to bind specific regulatory DNA sequences in the promoter of its target genes, which are well conserved between S. cerevisiae and K. lactis. Oppositely, we found important differences between ScIrx1 and KlIxr1 affecting cellular responses to cisplatin or cycloheximide in these yeasts, which could be dependent on specific and non-conserved domains present in these two proteins.
Keywords: IXR1; Kluyveromyces lactis; cisplatin sensitivity; dNTP pool; hypoxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

