Antibiotics Used in Empiric Treatment of Ocular Infections Trigger the Bacterial Rcs Stress Response System Independent of Antibiotic Susceptibility
- PMID: 34572615
- PMCID: PMC8470065
- DOI: 10.3390/antibiotics10091033
Antibiotics Used in Empiric Treatment of Ocular Infections Trigger the Bacterial Rcs Stress Response System Independent of Antibiotic Susceptibility
Abstract
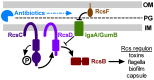
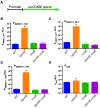
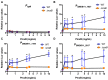
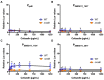
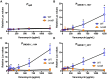
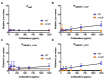
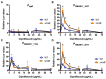
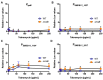
The Rcs phosphorelay is a bacterial stress response system that responds to envelope stresses and in turn controls several virulence-associated pathways, including capsule, flagella, and toxin biosynthesis, of numerous bacterial species. The Rcs system also affects antibiotic tolerance, biofilm formation, and horizontal gene transfer. The Rcs system of the ocular bacterial pathogen Serratia marcescens was recently demonstrated to influence ocular pathogenesis in a rabbit model of keratitis, with Rcs-defective mutants causing greater pathology and Rcs-activated strains demonstrating reduced inflammation. The Rcs system is activated by a variety of insults, including β-lactam antibiotics and polymyxin B. In this study, we developed three luminescence-based transcriptional reporters for Rcs system activity and used them to test whether antibiotics used for empiric treatment of ocular infections influence Rcs system activity in a keratitis isolate of S. marcescens. These included antibiotics to which the bacteria were susceptible and resistant. Results indicate that cefazolin, ceftazidime, polymyxin B, and vancomycin activate the Rcs system to varying degrees in an RcsB-dependent manner, whereas ciprofloxacin and tobramycin activated the promoter fusions, but in an Rcs-independent manner. Although minimum inhibitory concentration (MIC) analysis demonstrated resistance of the test bacteria to polymyxin B and vancomycin, the Rcs system was activated by sub-inhibitory concentrations of these antibiotics. Together, these data indicate that a bacterial stress system that influences numerous pathogenic phenotypes and drug-tolerance is influenced by different classes of antibiotics despite the susceptibility status of the bacterium.
Keywords: Enterobacterales; antibiotic; bacteria; cornea; infection; keratitis; stress response system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hume E.B., Willcox M.D. Emergence of Serratia marcescens as an ocular surface pathogen. Arch. Soc. Esp. Oftalmol. 2004;79:475–477. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

