Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies
- PMID: 34572661
- PMCID: PMC8468123
- DOI: 10.3390/antibiotics10091079
Mucormycosis in Indian COVID-19 Patients: Insight into Its Patho-Genesis, Clinical Manifestation, and Management Strategies
Abstract
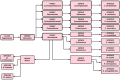
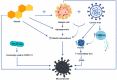
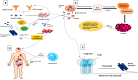
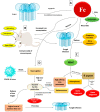
Mucormycosis in patients who have COVID-19 or who are otherwise immunocompromised has become a global problem, causing significant morbidity and mortality. Infection is debilitating and fatal, leading to loss of organs and emotional trauma. Radiographic manifestations are not specific, but diagnosis can be made through microscopic examination of materials collected from necrotic lesions. Treatment requires multidisciplinary expertise, as the fungus enters through the eyes and nose and may even reach the brain. Use of the many antifungal drugs available is limited by considerations of resistance and toxicity, but nanoparticles can overcome such limitations by reducing toxicity and increasing bioavailability. The lipid formulation of amphotericin-B (liposomal Am-B) is the first-line treatment for mucormycosis in COVID-19 patients, but its high cost and low availability have prompted a shift toward surgery, so that surgical debridement to remove all necrotic lesions remains the hallmark of effective treatment of mucormycosis in COVID-19. This review highlights the pathogenesis, clinical manifestation, and management of mucormycosis in patients who have COVID-19.
Keywords: COVID-19; amphotericin-B; mucormycosis; nanoparticles; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Virulence traits and novel drug delivery strategies for mucormycosis post-COVID-19: a comprehensive review.Front Immunol. 2023 Sep 25;14:1264502. doi: 10.3389/fimmu.2023.1264502. eCollection 2023. Front Immunol. 2023. PMID: 37818370 Free PMC article. Review.
-
Mucormycosis threats: A systemic review.J Basic Microbiol. 2023 Feb;63(2):119-127. doi: 10.1002/jobm.202200334. Epub 2022 Nov 4. J Basic Microbiol. 2023. PMID: 36333107
-
[Expert consensus on diagnosis and treatment of severe COVID-19 associated pulmonary aspergillosis and mucormycosis].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Jan 12;47(1):10-23. doi: 10.3760/cma.j.cn112147-20230823-00098. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38062689 Chinese.
-
Orbital Mucormycosis: Understanding the Deadly Fungus Sweeping the Globe.Cureus. 2023 Jun 26;15(6):e41010. doi: 10.7759/cureus.41010. eCollection 2023 Jun. Cureus. 2023. PMID: 37519583 Free PMC article.
-
Is mucormycosis the end? A comprehensive management of orbit in COVID associated rhino-orbital-cerebral mucormycosis: preserving the salvageable.Eur Arch Otorhinolaryngol. 2023 Feb;280(2):819-827. doi: 10.1007/s00405-022-07620-3. Epub 2022 Sep 2. Eur Arch Otorhinolaryngol. 2023. PMID: 36053359 Free PMC article.
Cited by
-
Rhino-orbital-cerebral mucormycosis as a complication of coronavirus disease 2019.World J Virol. 2022 Sep 25;11(5):293-299. doi: 10.5501/wjv.v11.i5.293. World J Virol. 2022. PMID: 36188746 Free PMC article. Review.
-
Xenosiderophores: bridging the gap in microbial iron acquisition strategies.World J Microbiol Biotechnol. 2025 Feb 13;41(2):69. doi: 10.1007/s11274-025-04287-w. World J Microbiol Biotechnol. 2025. PMID: 39939429 Review.
-
Mucormycosis in pre-COVID-19 and COVID-19 era: A study of prevalence, risk factors and clinical features.Laryngoscope Investig Otolaryngol. 2022 Sep 7;7(5):1343-50. doi: 10.1002/lio2.899. Online ahead of print. Laryngoscope Investig Otolaryngol. 2022. PMID: 36249085 Free PMC article.
-
Study of Management of Mucormycosis in COVID and Post-COVID Patients with Liposomal Amphotericin B its Outcome and Complications in a dedicated COVID Hospital from Eastern India.Niger Med J. 2025 Jun 16;66(2):746-753. doi: 10.71480/nmj.v66i2.666. eCollection 2025 Mar-Apr. Niger Med J. 2025. PMID: 40703866 Free PMC article.
-
Mucormycosis: A Rare disease to Notifiable Disease.Braz J Microbiol. 2024 Jun;55(2):1065-1081. doi: 10.1007/s42770-024-01315-z. Epub 2024 Apr 1. Braz J Microbiol. 2024. PMID: 38561499 Free PMC article. Review.
References
-
- Perfect J.R., Mourad A. Antifungal Therapy. CRC Press; Boca Raton, FL, USA: 2019. Management of mucormycoses; pp. 357–362.
-
- Mohanty A., Gupta P., Varshney S., Kabi A., Angral S. Breaking the mold: A brief review on the diagnostic and treatment approaches of mucormycosis. Int. J. Otorhinolaryngol. Head Neck Surg. 2021;7:1. doi: 10.18203/issn.2454-5929.ijohns20212336. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

