The Biology of Colicin M and Its Orthologs
- PMID: 34572691
- PMCID: PMC8469651
- DOI: 10.3390/antibiotics10091109
The Biology of Colicin M and Its Orthologs
Abstract
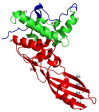
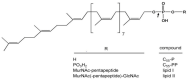
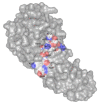
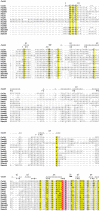
The misuse of antibiotics during the last decades led to the emergence of multidrug resistant pathogenic bacteria. This phenomenon constitutes a major public health issue. Consequently, the discovery of new antibacterials in the short term is crucial. Colicins, due to their antibacterial properties, thus constitute good candidates. These toxin proteins, produced by E. coli to kill enteric relative competitors, exhibit cytotoxicity through ionophoric activity or essential macromolecule degradation. Among the 25 colicin types known to date, colicin M (ColM) is the only one colicin interfering with peptidoglycan biosynthesis. Accordingly, ColM develops its lethal activity in E. coli periplasm by hydrolyzing the last peptidoglycan precursor, lipid II, into two dead-end products, thereby leading to cell lysis. Since the discovery of its unusual mode of action, several ColM orthologs have also been identified based on sequence alignments; all of the characterized ColM-like proteins display the same enzymatic activity of lipid II degradation and narrow antibacterial spectra. This publication aims at being an exhaustive review of the current knowledge on this new family of antibacterial enzymes as well as on their potential use as food preservatives or therapeutic agents.
Keywords: antibacterials; colicin M; colicins; lipid II; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

