Predictors of Voriconazole Trough Concentrations in Patients with Child-Pugh Class C Cirrhosis: A Prospective Study
- PMID: 34572712
- PMCID: PMC8470058
- DOI: 10.3390/antibiotics10091130
Predictors of Voriconazole Trough Concentrations in Patients with Child-Pugh Class C Cirrhosis: A Prospective Study
Abstract
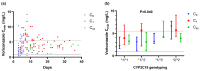
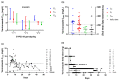
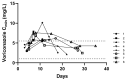
This prospective observational study aimed to clinically describe voriconazole administrations and trough concentrations in patients with Child-Pugh class C and to investigate the variability of trough concentration. A total of 144 voriconazole trough concentrations from 43 Child-Pugh class C patients were analyzed. The majority of patients (62.8%) received adjustments. The repeated measured trough concentration was higher than the first and final ones generally (median, 4.33 vs. 2.99, 3.90 mg/L). Eight patients with ideal initial concentrations later got supratherapeutic with no adjusted daily dose, implying accumulation. There was a significant difference in concentrations among the six groups by daily dose (p = 0.006). The bivariate correlation analysis showed that sex, CYP2C19 genotyping, daily dose, prothrombin time activity, international normalized ratio, platelet, and Model for end-stage liver disease score were significant factors for concentration. Subsequently, the first four factors mentioned above entered into a stepwise multiple linear regression model (variance inflation factor <5), implying that CYP2C19 testing makes sense for precision medicine of Child-Pugh class C cirrhosis patients. The equation fits well and explains the 34.8% variety of concentrations (R2 = 0.348). In conclusion, it needs more cautious administration clinically due to no recommendation for Child-Pugh class C patients in the medication label. The adjustment of the administration regimen should be mainly based on the results of repeated therapeutic drug monitoring.
Keywords: CYP2C19; Child–Pugh C cirrhosis; administration; trough concentrations; voriconazole.
Conflict of interest statement
The authors have indicated that they have no conflict of interest regarding the content of this article.
Figures

Similar articles
-
Necessity for a Significant Maintenance Dosage Reduction of Voriconazole in Patients with Severe Liver Cirrhosis (Child-Pugh Class C).Biol Pharm Bull. 2018 Jul 1;41(7):1112-1118. doi: 10.1248/bpb.b18-00164. Epub 2018 May 11. Biol Pharm Bull. 2018. PMID: 29760306
-
Therapeutic drug monitoring and safety of voriconazole therapy in patients with Child-Pugh class B and C cirrhosis: A multicenter study.Int J Infect Dis. 2018 Jul;72:49-54. doi: 10.1016/j.ijid.2018.05.009. Epub 2018 May 21. Int J Infect Dis. 2018. PMID: 29793038
-
A retrospective, multicenter study of voriconazole trough concentrations and safety in patients with Child-Pugh class C cirrhosis.J Clin Pharm Ther. 2018 Dec;43(6):849-854. doi: 10.1111/jcpt.12724. Epub 2018 Jun 11. J Clin Pharm Ther. 2018. PMID: 29893015
-
Voriconazole: a review of adjustment programs guided by therapeutic drug monitoring.Front Pharmacol. 2024 Dec 6;15:1439586. doi: 10.3389/fphar.2024.1439586. eCollection 2024. Front Pharmacol. 2024. PMID: 39712496 Free PMC article. Review.
-
Effect of Therapeutic Drug Monitoring and Cytochrome P450 2C19 Genotyping on Clinical Outcomes of Voriconazole: A Systematic Review.Ann Pharmacother. 2021 Apr;55(4):509-529. doi: 10.1177/1060028020948174. Epub 2020 Aug 8. Ann Pharmacother. 2021. PMID: 32772568
Cited by
-
Enhancing voriconazole therapy in liver dysfunction: exploring administration schemes and predictive factors for trough concentration and efficacy.Front Pharmacol. 2024 Jan 4;14:1323755. doi: 10.3389/fphar.2023.1323755. eCollection 2023. Front Pharmacol. 2024. PMID: 38239188 Free PMC article.
-
Impact of extracorporeal membrane oxygenation on voriconazole plasma concentrations: A retrospective study.Front Pharmacol. 2022 Aug 17;13:972585. doi: 10.3389/fphar.2022.972585. eCollection 2022. Front Pharmacol. 2022. PMID: 36059951 Free PMC article.
-
Factors Affecting Voriconazole Trough Concentration and Optimal Maintenance Voriconazole Dose in Chinese Children.Antibiotics (Basel). 2021 Dec 16;10(12):1542. doi: 10.3390/antibiotics10121542. Antibiotics (Basel). 2021. PMID: 34943754 Free PMC article.
-
Recent Advances in Therapeutic Drug Monitoring of Voriconazole, Mycophenolic Acid, and Vancomycin: A Literature Review of Pediatric Studies.Pharmaceutics. 2021 Nov 23;13(12):1991. doi: 10.3390/pharmaceutics13121991. Pharmaceutics. 2021. PMID: 34959272 Free PMC article. Review.
-
Associated factors with voriconazole plasma concentration: a systematic review and meta-analysis.Front Pharmacol. 2024 Aug 23;15:1368274. doi: 10.3389/fphar.2024.1368274. eCollection 2024. Front Pharmacol. 2024. PMID: 39246651 Free PMC article.
References
-
- Wan S.Z., Nie Y., Zhang Y., Liu C., Zhu X. Assessing the Prognostic Performance of the Child-Pugh, Model for End-Stage Liver Disease, and Albumin-Bilirubin Scores in Patients with Decompensated Cirrhosis: A Large Asian Cohort from Gastroenterology Department. Dis. Markers. 2020;2020:5193028. doi: 10.1155/2020/5193028. - DOI - PMC - PubMed
-
- Theocharidou E., Agarwal B., Jeffrey G., Jalan R., Harrison D., Burroughs A.K., Kibbler C.C. Early invasive fungal infections and colonization in patients with cirrhosis admitted to the intensive care unit. Clin. Microbiol. Infect. 2016;22:189.e181–189.e187. doi: 10.1016/j.cmi.2015.10.020. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

