Detection of Multidrug-Resistant Enterobacterales-From ESBLs to Carbapenemases
- PMID: 34572722
- PMCID: PMC8465816
- DOI: 10.3390/antibiotics10091140
Detection of Multidrug-Resistant Enterobacterales-From ESBLs to Carbapenemases
Abstract
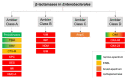
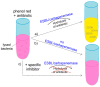
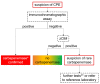
Multidrug-resistant Enterobacterales (MDRE) are an emerging threat to global health, leading to rising health care costs, morbidity and mortality. Multidrug-resistance is commonly caused by different β-lactamases (e.g., ESBLs and carbapenemases), sometimes in combination with other resistance mechanisms (e.g., porin loss, efflux). The continuous spread of MDRE among patients in hospital settings and the healthy population require adjustments in healthcare management and routine diagnostics. Rapid and reliable detection of MDRE infections as well as gastrointestinal colonization is key to guide therapy and infection control measures. However, proper implementation of these strategies requires diagnostic methods with short time-to-result, high sensitivity and specificity. Therefore, research on new techniques and improvement of already established protocols is inevitable. In this review, current methods for detection of MDRE are summarized with focus on culture based and molecular techniques, which are useful for the clinical microbiology laboratory.
Keywords: CPE; ESBL; carbapenemase; detection methods; multidrug resistance; preanalytical parameters.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

