The Role of p53 in Progression of Cutaneous Squamous Cell Carcinoma
- PMID: 34572732
- PMCID: PMC8466956
- DOI: 10.3390/cancers13184507
The Role of p53 in Progression of Cutaneous Squamous Cell Carcinoma
Abstract
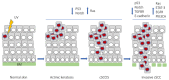
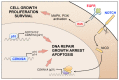
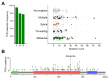
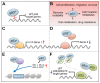
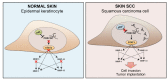
Skin cancers are the most common types of cancer worldwide, and their incidence is increasing. Melanoma, basal cell carcinoma (BCC), and cutaneous squamous cell carcinoma (cSCC) are the three major types of skin cancer. Melanoma originates from melanocytes, whereas BCC and cSCC originate from epidermal keratinocytes and are therefore called keratinocyte carcinomas. Chronic exposure to ultraviolet radiation (UVR) is a common risk factor for skin cancers, but they differ with respect to oncogenic mutational profiles and alterations in cellular signaling pathways. cSCC is the most common metastatic skin cancer, and it is associated with poor prognosis in the advanced stage. An important early event in cSCC development is mutation of the TP53 gene and inactivation of the tumor suppressor function of the tumor protein 53 gene (TP53) in epidermal keratinocytes, which then leads to accumulation of additional oncogenic mutations. Additional genomic and proteomic alterations are required for the progression of premalignant lesion, actinic keratosis, to invasive and metastatic cSCC. Recently, the role of p53 in the invasion of cSCC has also been elucidated. In this review, the role of p53 in the progression of cSCC and as potential new therapeutic target for cSCC will be discussed.
Keywords: cancer; p53; skin; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
New perspectives on role of tumor microenvironment in progression of cutaneous squamous cell carcinoma.Cell Tissue Res. 2016 Sep;365(3):691-702. doi: 10.1007/s00441-016-2457-z. Epub 2016 Jul 14. Cell Tissue Res. 2016. PMID: 27411692 Review.
-
Matrix metalloproteinases in keratinocyte carcinomas.Exp Dermatol. 2021 Jan;30(1):50-61. doi: 10.1111/exd.14183. Epub 2020 Sep 17. Exp Dermatol. 2021. PMID: 32869366 Free PMC article. Review.
-
Premalignant lesions, basal cell carcinoma and melanoma in patients with cutaneous squamous cell carcinoma.Arch Dermatol Res. 2021 Dec;313(10):879-884. doi: 10.1007/s00403-020-02114-w. Epub 2020 Aug 9. Arch Dermatol Res. 2021. PMID: 32772261 Free PMC article.
-
Targeted deep sequencing reveals genomic alterations of actinic keratosis/cutaneous squamous cell carcinoma in situ and cutaneous squamous cell carcinoma.Exp Dermatol. 2023 Apr;32(4):447-456. doi: 10.1111/exd.14730. Epub 2022 Dec 26. Exp Dermatol. 2023. PMID: 36533870
-
[ASSOCIATION OF SKIN PHOTOTYPE AND UV EXPOSURE WITH EXPRESSION OF HER RECEPTORS, Ki67 AND p53 IN PATIENTS WITH CUTANEOUS SQUAMOUS CELL CARCINOMA].Acta Med Croatica. 2015;69(5):431-8. Acta Med Croatica. 2015. PMID: 29087088 Croatian.
Cited by
-
Comparative Study of Cutaneous Squamous Cell Carcinogenesis in Different Hairless Murine Models.Cancers (Basel). 2024 Oct 21;16(20):3546. doi: 10.3390/cancers16203546. Cancers (Basel). 2024. PMID: 39456640 Free PMC article.
-
New Insights into the Role of PPARγ in Skin Physiopathology.Biomolecules. 2024 Jun 19;14(6):728. doi: 10.3390/biom14060728. Biomolecules. 2024. PMID: 38927131 Free PMC article. Review.
-
Therapeutic Approaches for Non-Melanoma Skin Cancer: Standard of Care and Emerging Modalities.Int J Mol Sci. 2024 Jun 27;25(13):7056. doi: 10.3390/ijms25137056. Int J Mol Sci. 2024. PMID: 39000164 Free PMC article. Review.
-
Super Enhancer-Regulated LINC00094 (SERLOC) Upregulates the Expression of MMP-1 and MMP-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma.Cancers (Basel). 2022 Aug 17;14(16):3980. doi: 10.3390/cancers14163980. Cancers (Basel). 2022. PMID: 36010973 Free PMC article.
-
From actinic keratosis to cutaneous squamous cell carcinoma: the key pathogenesis and treatments.Front Immunol. 2025 Jan 24;16:1518633. doi: 10.3389/fimmu.2025.1518633. eCollection 2025. Front Immunol. 2025. PMID: 39925808 Free PMC article. Review.
References
-
- Venables Z.C., Nijsten T., Wong K.F., Autier P., Broggio J., Deas A., Harwood C.A., Hollestein L.M., Langan S.M., Morgan E., et al. Epidemiology of basal and cutaneous squamous cell carcinoma in the U.K. 2013–15: A cohort study. Br. J. Dermatol. 2019;181:474–482. doi: 10.1111/bjd.17873. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

