PD-1/PD-L1 Checkpoints and Resveratrol: A Controversial New Way for a Therapeutic Strategy
- PMID: 34572736
- PMCID: PMC8467857
- DOI: 10.3390/cancers13184509
PD-1/PD-L1 Checkpoints and Resveratrol: A Controversial New Way for a Therapeutic Strategy
Abstract
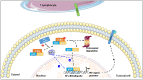
Immune checkpoints refer to a range of immunoregulatory molecules that modulate the immune response. For example, proteins expressed at the surface of T-cells (including PD-1 and CTLA-4) and their ligands (PD-L1 and B7-1/B7-2, respectively), expressed by cancer cells and antigen-presenting cells, are needed to prevent excessive immune responses. However, they dampen anti-tumor immunity by limiting T-cell activity, making them promising therapeutic targets in cancer. Although immunotherapies using checkpoint blocking/neutralizing antibodies targeting PD-L1 or PD-1 have proven their superiority over conventional chemotherapies or targeted therapies by enhancing T-cell-mediated anti-tumor immunity, some limitations have emerged. These include a relatively low rate of "responders" (<50%; irrespective of cancer type), the high cost of injections, and a rare risk of hyper-progression. For clinicians, the current challenge is thus to improve the existing therapies, potentially through combinatory approaches. Polyphenols such as resveratrol (RSV), a trihydroxystilbene found in various plants and an adjuvant in numerous nutraceuticals, have been proposed as potential therapeutic targets. Beyond its well-known pleiotropic effects, RSV affects PD-L1 and PD-1 expression as well as PD-L1 subcellular localization and post-translational modifications, which we review here. We also summarize the consequences of PD-1/PD-L1 signaling, the modalities of their blockade in the context of cancer, and the current status and limitations of these immunotherapies. Finally, we discuss their potential use in combination with chemotherapies, and, using RSV as a model, we propose polyphenols as adjuvants to enhance the efficacy of anti-PD-1/anti-PD-L1 immunotherapies.
Keywords: PD-1; PD-L1; immunotherapy; polyphenols; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity.Aging (Albany NY). 2020 Jan 4;12(1):8-34. doi: 10.18632/aging.102646. Epub 2020 Jan 4. Aging (Albany NY). 2020. PMID: 31901900 Free PMC article.
-
Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma.Cancer Med. 2016 Aug;5(8):1810-20. doi: 10.1002/cam4.754. Epub 2016 Jun 12. Cancer Med. 2016. PMID: 27292320 Free PMC article.
-
MicroRNA-326 attenuates immune escape and prevents metastasis in lung adenocarcinoma by targeting PD-L1 and B7-H3.Cell Death Discov. 2021 Jun 15;7(1):145. doi: 10.1038/s41420-021-00527-8. Cell Death Discov. 2021. PMID: 34131111 Free PMC article.
-
Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy.J Hematol Oncol. 2016 May 27;9(1):47. doi: 10.1186/s13045-016-0277-y. J Hematol Oncol. 2016. PMID: 27234522 Free PMC article. Review.
Cited by
-
The Impact of Proton Pump Inhibitors on the Efficacy of Immune Checkpoint Inhibitor Combinations in Patients with HBV-Associated Advanced Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2024 Jul 4;11:1311-1321. doi: 10.2147/JHC.S464033. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 38979082 Free PMC article.
-
B7 family protein glycosylation: Promising novel targets in tumor treatment.Front Immunol. 2022 Dec 6;13:1088560. doi: 10.3389/fimmu.2022.1088560. eCollection 2022. Front Immunol. 2022. PMID: 36561746 Free PMC article. Review.
-
Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer.Nutrients. 2024 Nov 29;16(23):4143. doi: 10.3390/nu16234143. Nutrients. 2024. PMID: 39683540 Free PMC article. Review.
-
Resveratrol contributes to NK cell-mediated breast cancer cytotoxicity by upregulating ULBP2 through miR-17-5p downmodulation and activation of MINK1/JNK/c-Jun signaling.Front Immunol. 2025 Feb 3;16:1515605. doi: 10.3389/fimmu.2025.1515605. eCollection 2025. Front Immunol. 2025. PMID: 39963142 Free PMC article.
-
Characterization of Cell Death Induced by Imine Analogs of Trans-Resveratrol: Induction of Mitochondrial Dysfunction and Overproduction of Reactive Oxygen Species Leading to, or Not, Apoptosis without the Increase in the S-Phase of the Cell Cycle.Molecules. 2023 Apr 3;28(7):3178. doi: 10.3390/molecules28073178. Molecules. 2023. PMID: 37049947 Free PMC article.
References
-
- Zhang L., Conejo-Garcia J.R., Katsaros D., Gimotty P.A., Massobrio M., Regnani G., Makrigiannakis A., Gray H., Schlienger K., Liebman M.N., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. - DOI - PubMed
-
- Wu C.Y., Chen D.Y., Shen J.L., Ho H.J., Chen C.C., Kuo K.N., Liu H.N., Chang Y.T., Chen Y.J. The risk of cancer in patients with rheumatoid arthritis taking tumor necrosis factor antagonists: A nationwide cohort study. Arthritis Res. Ther. 2014;16:449. doi: 10.1186/s13075-014-0449-5. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

