Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions
- PMID: 34572758
- PMCID: PMC8464971
- DOI: 10.3390/cancers13184530
Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions
Abstract
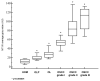
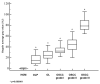
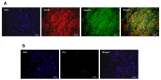
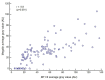
This study aimed to assess the relationship and possible interactions between metallothioneins (MTs) and megalin (LRP-2) in different grades of oral squamous cell carcinoma (OSCC) and premalignant lesions of the oral mucosa (oral leukoplakia and oral lichen planus). The study included archived samples of 114 patients and control subjects. Protein expression was examined by immunohistochemistry and immunofluorescence, and staining quantification was performed by ImageJ software. Protein interaction in cancer tissue was tested and visualized by proximity ligation assay. Mann-Whitney and Kruskal-Wallis tests were used to determine the significance of differences between each group, whereas Pearson correlation coefficient was performed to test correlation. Expression of both proteins differed significantly between each group showing the same pattern of gradual increasing from oral lichen planus to poorly differentiated OSCC. Moreover, MTs and megalin were found to co-express and interact in cancer tissue, and their expression positively correlated within the overall study group. Findings of prominent nuclear and chromosomal megalin expression suggest that it undergoes regulated intramembrane proteolysis upon MTs binding, indicating its ability to directly affect gene expression and cellular division in cancer tissue. The data obtained point to the onco-driving potential of MTs-megalin interaction.
Keywords: interaction; lichen planus; megalin; metallothioneins; oral leukoplakia; oral squamous cell carcinoma; regulated intramembrane proteolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Comparative evaluation of survivin expression in leukoplakia, lichen planus, and oral squamous cell carcinoma: An immunohistochemical study.J Cancer Res Ther. 2020 Apr-Jun;16(3):569-574. doi: 10.4103/jcrt.JCRT_421_19. J Cancer Res Ther. 2020. PMID: 32719269
-
Cathepsin-B and caveolin-1 gene expressions in oral lichen planus and oral squamous cell carcinoma.Mol Biol Rep. 2022 Apr;49(4):2945-2951. doi: 10.1007/s11033-022-07115-8. Epub 2022 Feb 9. Mol Biol Rep. 2022. PMID: 35138525
-
Prevalence and diagnostic significance of p16, p53 expression in lichen planus as a potential premalignant lesion in oral squamous cell carcinoma.J Oral Maxillofac Pathol. 2024 Jan-Mar;28(1):56-61. doi: 10.4103/jomfp.jomfp_427_23. Epub 2024 Apr 15. J Oral Maxillofac Pathol. 2024. PMID: 38800439 Free PMC article.
-
Evaluation of Potential Risk Factors that contribute to Malignant Transformation of Oral Lichen Planus: A Literature Review.J Contemp Dent Pract. 2016 Aug 1;17(8):692-701. doi: 10.5005/jp-journals-10024-1914. J Contemp Dent Pract. 2016. PMID: 27659090 Review.
-
Molecular genetics of premalignant oral lesions.Oral Dis. 2007 Mar;13(2):126-33. doi: 10.1111/j.1601-0825.2006.01349.x. Oral Dis. 2007. PMID: 17305612 Review.
Cited by
-
Multiplex Tissue Imaging: Spatial Revelations in the Tumor Microenvironment.Cancers (Basel). 2022 Jun 28;14(13):3170. doi: 10.3390/cancers14133170. Cancers (Basel). 2022. PMID: 35804939 Free PMC article. Review.
-
Disruption of H3K36 methylation provokes cellular plasticity to drive aberrant glandular formation and squamous carcinogenesis.Dev Cell. 2024 Jan 22;59(2):187-198.e7. doi: 10.1016/j.devcel.2023.12.007. Epub 2024 Jan 9. Dev Cell. 2024. PMID: 38198888 Free PMC article.
-
Megalin Expression in Primary Oral Squamous Cell Carcinoma Is Associated with the Presence of Lymph Node Metastases, Vascular Invasion, and Lower Overall Survival.Curr Issues Mol Biol. 2023 Mar 24;45(4):2757-2766. doi: 10.3390/cimb45040180. Curr Issues Mol Biol. 2023. PMID: 37185704 Free PMC article.
References
-
- Merlos Rodrigo M.A., Jimenez Jimemez A.M., Haddad Y., Bodoor K., Adam P., Krizkova S., Heger Z., Adam V. Metallothionein isoforms as double agents—Their roles in carcinogenesis, cancer progression and chemoresistance. Drug Resist. Updat. 2020;52:100691. doi: 10.1016/j.drup.2020.100691. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

