Blinded Independent Central Review (BICR) in New Therapeutic Lung Cancer Trials
- PMID: 34572761
- PMCID: PMC8465869
- DOI: 10.3390/cancers13184533
Blinded Independent Central Review (BICR) in New Therapeutic Lung Cancer Trials
Abstract
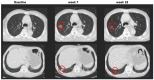
Background: Double reads in blinded independent central reviews (BICRs) are recommended to control the quality of trials but they are prone to discordances. We analyzed inter-reader discordances in a pool of lung cancer trials using RECIST 1.1.
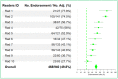
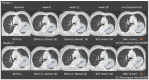
Methods: We analyzed six lung cancer BICR trials that included 1833 patients (10,684 time points) involving 17 radiologists. We analyzed the rate of discrepancy of each trial at the time-point and patient levels as well as testing inter-trial differences. The analysis of adjudication made it possible to compute the readers' endorsement rates, the root causes of adjudications, and the proportions of "errors" versus "medically justifiable differences".
Results: The trials had significantly different discrepancy rates both at the time-point (average = 34.3%) and patient (average = 59.2%) levels. When considering only discrepancies for progressive disease, homogeneous discrepancy rates were found with an average of 32.9%, while readers' endorsement rates ranged between 27.7% and 77.8%. Major causes of adjudication were different per trial, with medically justifiable differences being the most common, triggering 74.2% of total adjudications.
Conclusions: We provide baseline performances for monitoring reader performance in trials with double reads. Intelligent reading system implementation along with appropriate reader training and monitoring are solutions that could mitigate a large portion of the commonly encountered reading errors.
Keywords: RECIST 1.1; blinded independent central review; clinical trial; lung cancer.
Conflict of interest statement
All authors are employees of Median Technologies.
Figures


References
-
- Pilz L.R., Manegold C., Schmid-Bindert G. Statistical considerations and endpoints for clinical lung cancer studies: Can progression free survival (PFS) substitute overall survival (OS) as a valid endpoint in clinical trials for advanced nonsmall- cell lung cancer? Transl. Lung Cancer Res. 2012;1:26–35. doi: 10.3978/j.issn.2218-6751.2011.12.08. - DOI - PMC - PubMed
-
- Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. - DOI - PubMed
LinkOut - more resources
Full Text Sources

