Targeting Autophagy Triggers Apoptosis and Complements the Action of Venetoclax in Chronic Lymphocytic Leukemia Cells
- PMID: 34572784
- PMCID: PMC8466897
- DOI: 10.3390/cancers13184557
Targeting Autophagy Triggers Apoptosis and Complements the Action of Venetoclax in Chronic Lymphocytic Leukemia Cells
Abstract
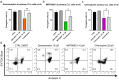
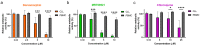
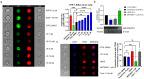
Continuous treatment of patients with chronic lymphocytic leukemia (CLL) with venetoclax, an antagonist of the anti-apoptotic protein Bcl-2, can result in resistance, which highlights the need for novel targets to trigger cell death in CLL. Venetoclax also induces autophagy by perturbing the Bcl-2/Beclin-1 complex, so autophagy might represent a target in CLL. Diverse autophagy inhibitors were assessed for cytotoxic activities against patient-derived CLL cells. The AMPK inhibitor dorsomorphin, the ULK1/2 inhibitor MRT68921, and the autophagosome-lysosome fusion inhibitor chloroquine demonstrated concentration-dependent and time-dependent cytotoxicity against CLL cells, even in those from hard-to-treat patients who carried del(11q) and del(17p). Dorsomorphin and MRT68921 but not chloroquine triggered caspase-dependent cell death. According to the metabolic activities of CLL cells and PBMCs following treatments with 10 µM dorsomorphin (13% vs. 84%), 10 µM MRT68921 (7% vs. 78%), and 25 µM chloroquine (41% vs. 107%), these autophagy inhibitors are selective toward CLL cells. In these CLL cells, venetoclax induced autophagy, and addition of dorsomorphin, MRT68921, or chloroquine showed potent synergistic cytotoxicities. Additionally, MRT68921 alone induced G2 arrest, but when combined with venetoclax, it triggered caspase-dependent cytotoxicity. These data provide the rationale to target autophagy and for autophagy inhibitors as potential treatments for patients with CLL.
Keywords: AMPK/ULK1; autophagy; chronic lymphocytic leukemia; drug resistance; targeted therapy; venetoclax.
Conflict of interest statement
Authors H.P. and M.Š. have been involved as consultants for Abbvie. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

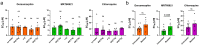

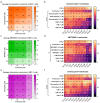
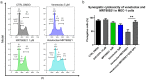
References
-
- Venclyxto, INN-Venetoclax. [(accessed on 10 April 2021)]. Available online: https://www.ema.europa.eu/en/documents/product-information/venclyxto-epa....
Grants and funding
LinkOut - more resources
Full Text Sources

