Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin
- PMID: 34572794
- PMCID: PMC8469571
- DOI: 10.3390/cancers13184566
Therapeutic Targeting of Acute Myeloid Leukemia by Gemtuzumab Ozogamicin
Abstract
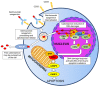
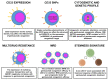
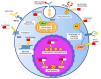
Acute myeloid leukemia (AML) is a complex hematological malignancy characterized by genetic and clinical heterogeneity and high mortality. Despite the recent introduction of novel pharmaceutical agents in hemato-oncology, few advancements have been made in AML for decades. In the last years, the therapeutic options have rapidly changed, with the approval of innovative compounds that provide new opportunities, together with new challenges for clinicians: among them, on 1 September, 2017 the Food and Drug Administration granted approval for Gemtuzumab Ozogamicin (GO) in combination with daunorubicin and cytarabine for the treatment of adult patients affected by newly diagnosed CD33+ AML. Benefits of GO-based regimens were also reported in the pre- and post-transplantation settings. Moreover, several biomarkers of GO response have been suggested, including expression of CD33 and multidrug resistance genes, cytogenetic and molecular profiles, minimal residual disease and stemness signatures. Among them, elevated CD33 expression on blast cells and non-adverse cytogenetic or molecular risk represent largely validated predictors of good response.
Keywords: CD33; acute myeloid leukemia; biomarkers; gemtuzumab ozogamicin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
FDA Approval: Gemtuzumab Ozogamicin for the Treatment of Adults with Newly Diagnosed CD33-Positive Acute Myeloid Leukemia.Clin Cancer Res. 2018 Jul 15;24(14):3242-3246. doi: 10.1158/1078-0432.CCR-17-3179. Epub 2018 Feb 23. Clin Cancer Res. 2018. PMID: 29476018
-
Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia.Clin Cancer Res. 2001 Jun;7(6):1490-6. Clin Cancer Res. 2001. PMID: 11410481
-
Biomarkers of Gemtuzumab Ozogamicin Response for Acute Myeloid Leukemia Treatment.Int J Mol Sci. 2020 Aug 6;21(16):5626. doi: 10.3390/ijms21165626. Int J Mol Sci. 2020. PMID: 32781546 Free PMC article. Review.
-
FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia.Oncologist. 2018 Sep;23(9):1103-1108. doi: 10.1634/theoncologist.2017-0604. Epub 2018 Apr 12. Oncologist. 2018. PMID: 29650683 Free PMC article.
-
Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia.Expert Rev Clin Pharmacol. 2018 Jun;11(6):549-559. doi: 10.1080/17512433.2018.1478725. Epub 2018 Jun 11. Expert Rev Clin Pharmacol. 2018. PMID: 29787320 Free PMC article. Review.
Cited by
-
Nanotechnology-assisted combination drug delivery: a progressive approach for the treatment of acute myeloid leukemia.Ther Deliv. 2024;15(11):893-910. doi: 10.1080/20415990.2024.2394012. Epub 2024 Sep 13. Ther Deliv. 2024. PMID: 39268925 Review.
-
Regulatory considerations in the design, development and quality of monoclonal antibodies and related products for the diagnosis and treatment of cancer.Front Oncol. 2024 Apr 30;14:1379738. doi: 10.3389/fonc.2024.1379738. eCollection 2024. Front Oncol. 2024. PMID: 38746685 Free PMC article. Review.
-
Update on the role of gemtuzumab-ozogamicin in the treatment of acute myeloid leukemia.Ther Adv Hematol. 2023 Feb 9;14:20406207231154708. doi: 10.1177/20406207231154708. eCollection 2023. Ther Adv Hematol. 2023. PMID: 36845850 Free PMC article. Review.
-
Leukemia-Derived Dendritic Cells Induce Anti-Leukemic Effects Ex Vivo in AML Independently of Patients' Clinical and Biological Features.Int J Mol Sci. 2025 Feb 17;26(4):1700. doi: 10.3390/ijms26041700. Int J Mol Sci. 2025. PMID: 40004163 Free PMC article.
-
Multi-targeted, NOT gated CAR-T cells as a strategy to protect normal lineages for blood cancer therapy.Front Immunol. 2025 Mar 21;16:1493329. doi: 10.3389/fimmu.2025.1493329. eCollection 2025. Front Immunol. 2025. PMID: 40191207 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials