External Basic Hyperthermia Devices for Preclinical Studies in Small Animals
- PMID: 34572855
- PMCID: PMC8470307
- DOI: 10.3390/cancers13184628
External Basic Hyperthermia Devices for Preclinical Studies in Small Animals
Abstract
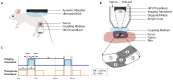
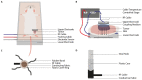
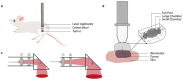
Preclinical studies have shown that application of mild hyperthermia (40-43 °C) is a promising adjuvant to solid tumor treatment. To improve preclinical testing, enhance reproducibility, and allow comparison of the obtained results, it is crucial to have standardization of the available methods. Reproducibility of methods in and between research groups on the same techniques is crucial to have a better prediction of the clinical outcome and to improve new treatment strategies (for instance with heat-sensitive nanoparticles). Here we provide a preclinically oriented review on the use and applicability of basic hyperthermia systems available for solid tumor thermal treatment in small animals. The complexity of these techniques ranges from a simple, low-cost water bath approach, irradiation with light or lasers, to advanced ultrasound and capacitive heating devices.
Keywords: hyperthermia; multimodal therapy; preclinical hyperthermia; small animals; standardization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

