Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases
- PMID: 34573081
- PMCID: PMC8470349
- DOI: 10.3390/antiox10091448
Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases
Abstract
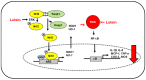
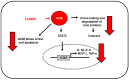
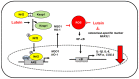
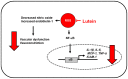
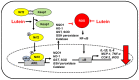
Lutein is a xanthophyll carotenoid obtained from various foods, such as dark green leafy vegetables and egg yolk. Lutein has antioxidant activity and scavenges reactive oxygen species such as singlet oxygen and lipid peroxy radicals. Oxidative stress activates inflammatory mediators, leading to the development of metabolic and inflammatory diseases. Thus, recent basic and clinical studies have investigated the anti-inflammatory effects of lutein based on its antioxidant activity and modulation of oxidant-sensitive inflammatory signaling pathways. Lutein suppresses activation of nuclear factor-kB and signal transducer and activator of transcription 3, and induction of inflammatory cytokines (interleukin-1β, interleukin-6, monocyte chemoattratant protein-1, tumor necrosis factor-α) and inflammatory enzymes (cyclooxygenase-2, inducible nitric oxide synthase). It also maintains the content of endogenous antioxidant (glutathione) and activates nuclear factor erythroid 2-related factor 2 (Nrf2) and Nrf2 signaling-related antioxidant enzymes (hemeoxygenase-1, NAD(P)H: quinone oxidoreductase 1, glutathione-s-transferase, glutathione peroxidase, superoxide dismutase, catalase). In this review, we have discussed the current knowledge regarding the anti-inflammatory function of lutein against inflammatory diseases in various organs, including neurodegenerative disorders, eye diseases, diabetic retinopathy, osteoporosis, cardiovascular diseases, skin diseases, liver injury, obesity, and colon diseases.
Keywords: inflammation; lutein; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

