Iron-Sulfur Cluster Biogenesis as a Critical Target in Cancer
- PMID: 34573089
- PMCID: PMC8465902
- DOI: 10.3390/antiox10091458
Iron-Sulfur Cluster Biogenesis as a Critical Target in Cancer
Abstract
Cancer cells preferentially accumulate iron (Fe) relative to non-malignant cells; however, the underlying rationale remains elusive. Iron-sulfur (Fe-S) clusters are critical cofactors that aid in a wide variety of cellular functions (e.g., DNA metabolism and electron transport). In this article, we theorize that a differential need for Fe-S biogenesis in tumor versus non-malignant cells underlies the Fe-dependent cell growth demand of cancer cells to promote cell division and survival by promoting genomic stability via Fe-S containing DNA metabolic enzymes. In this review, we outline the complex Fe-S biogenesis process and its potential upregulation in cancer. We also discuss three therapeutic strategies to target Fe-S biogenesis: (i) redox manipulation, (ii) Fe chelation, and (iii) Fe mimicry.
Keywords: cancer therapy; carcinogenesis; iron metabolism; iron–sulfur cluster biogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

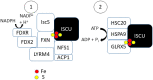





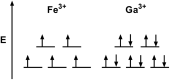
References
-
- Schwartz A.J., Goyert J.W., Solanki S., Kerk S.A., Chen B., Castillo C., Hsu P.P., Do B.T., Singhal R., Dame M.K., et al. Hepcidin sequesters iron to sustain nucleotide metabolism and mitochondrial function in colorectal cancer epithelial cells. Nat. Metab. 2021;3:969–982. doi: 10.1038/s42255-021-00406-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

