The Effect of Cannabis-Based Medicine on Neuropathic Pain and Spasticity in Patients with Multiple Sclerosis and Spinal Cord Injury: Study Protocol of a National Multicenter Double-Blinded, Placebo-Controlled Trial
- PMID: 34573231
- PMCID: PMC8465969
- DOI: 10.3390/brainsci11091212
The Effect of Cannabis-Based Medicine on Neuropathic Pain and Spasticity in Patients with Multiple Sclerosis and Spinal Cord Injury: Study Protocol of a National Multicenter Double-Blinded, Placebo-Controlled Trial
Abstract
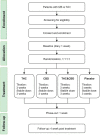
Disease or acquired damage to the central nervous system frequently causes disabling spasticity and central neuropathic pain (NP), both of which are frequent in multiple sclerosis (MS) and spinal cord injury (SCI). Patients with MS and SCI often request treatment with cannabis-based medicine (CBM). However, knowledge about effects, side effects, choice of active cannabinoids (Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) alone or in combination), and doses of CBM remains limited. Using a double-blind, parallel design in a national multicenter cohort, this study examines the effect of CBM on spasticity and NP. Patients are randomized to treatment with capsules containing either THC, CBD, THC and CBD, or placebo. Primary endpoints are patient-reported pain and spasticity on a numerical rating scale. Other endpoints include quality of life and sleep, depression and anxiety, and relief of pain and spasticity. Side-effects of CBM are described. In a sub-study, the pharmacodynamics (PD) and pharmacokinetics (PK) of oral capsule CBM are examined. We expect that the study will contribute to the literature by providing information on the effects and side-effects of CBD, THC, and the combination of the two for central neuropathic pain and spasticity. Furthermore, we will describe the PD/PK of THC and CBD in a patient population.
Keywords: CBD; THC; cannabinoids; cannabis-based medicine; medical cannabis; multiple sclerosis; neuropathic pain; numeric rating scale; spasticity; spinal cord injury.
Conflict of interest statement
E.A.S. and H.K. declare no competing interest. S.G. has received support for congress participation from Merck. K.S. received travel grants from TEVA, Biogen, Merck, and Novartis. J.S.H has received travel grants from Almirall and Roche. N.B.F has received consultancy fees from Merck, Almirall, NeuroPN, Vertex, and Novartis Pharma, and has undertaken consultancy work for Aarhus University with remunerated work for Biogen, Merz, and Confo Therapeutics. She has received grants from IMI2PainCare, an EU IMI 2 (Innovative medicines initiative) public-private consortium and the companies involved are: Grunenthal, Bayer, Eli Lilly, Esteve, and Teva. P.V.R. has received speaker honoraria from Biogen, Roche, Merck, Alexion, and Novartis; support for congress participation from Merck, Roche, and Sanofi; fees for serving on advisory boards from Bristol-Myers-Squibb, Merck, Roche, Novartis, Biogen, Sanofi, and Alexion. F.S. has served on scientific advisory boards for, served as consultant for, received support for congress participation, or received speaker honoraria from Alexion, Biogen, Bristol Myers Squibb, H. Lundbeck A/S, Merck, Novartis, Roche, and Sanofi Genzyme. His laboratory has received research support from Biogen, Merck, Novartis, Roche, and Sanofi Genzyme. R.M.H. has received support for congress participation from Merck and Ipsen. A.B.O. has served on scientific advisory boards for Biogen Idec, Novartis, and Sanofi Genzyme; has received research support from Novartis; has received speaker honoraria from Biogen, Novartis, and TEVA; and has received support for congress participation from, Merck, TEVA, Biogen, Roche, Novartis, and Sanofi Genzyme. T.S. has received research support to the MS clinic at Aarhus University Hospital from Merck, Alexion, Roche, Biogen, Novartis, and Sanofi.
References
-
- Finnerup N.B., Haroutounian S., Kamerman P., Baron R., Bennett D.L.H., Bouhassira D., Cruccu G., Freeman R., Hansson P., Nurmikko T., et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous