Pathogenic DNM1L Variant (1085G>A) Linked to Infantile Progressive Neurological Disorder: Evidence of Maternal Transmission by Germline Mosaicism and Influence of a Contemporary in cis Variant (1535T>C)
- PMID: 34573276
- PMCID: PMC8467311
- DOI: 10.3390/genes12091295
Pathogenic DNM1L Variant (1085G>A) Linked to Infantile Progressive Neurological Disorder: Evidence of Maternal Transmission by Germline Mosaicism and Influence of a Contemporary in cis Variant (1535T>C)
Abstract
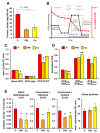
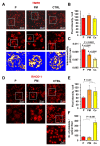
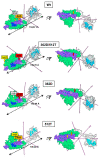
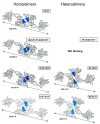
Mitochondria are dynamic organelles undergoing continuous fusion and fission with Drp1, encoded by the DNM1L gene, required for mitochondrial fragmentation. DNM1L dominant pathogenic variants lead to progressive neurological disorders with early exitus. Herein we report on the case of a boy affected by epileptic encephalopathy carrying two heterozygous variants (in cis) of the DNM1L gene: a pathogenic variant (PV) c.1085G>A (p.Gly362Asp) accompanied with a variant of unknown significance (VUS) c.1535T>C (p.Ile512Thr). Amplicon sequencing of the mother's DNA revealed the presence of the PV and VUS in 5% of cells, with the remaining cells presenting only VUS. Functional investigations performed on the patient and his mother's cells unveiled altered mitochondrial respiratory chain activities, network architecture and Ca2+ homeostasis as compared with healthy unrelated subjects' samples. Modelling Drp1 harbouring the two variants, separately or in combination, resulted in structural changes as compared with Wt protein. Considering the clinical history of the mother, PV transmission by a maternal germline mosaicism mechanism is proposed. Altered Drp1 function leads to changes in the mitochondrial structure and bioenergetics as well as in Ca2+ homeostasis. The novel VUS might be a modifier that synergistically worsens the phenotype when associated with the PV.
Keywords: DNM1L; Drp1; encephalopathy; genetic mosaicism; mitochondrial fission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

