Vitamins A and D and Zinc Affect the Leshmanicidal Activity of Canine Spleen Leukocytes
- PMID: 34573521
- PMCID: PMC8468882
- DOI: 10.3390/ani11092556
Vitamins A and D and Zinc Affect the Leshmanicidal Activity of Canine Spleen Leukocytes
Abstract
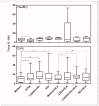
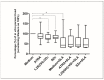
Canine leishmaniasis (CanL) is a chronic disease caused by Leishmania infantum, and the limitations of the current treatments have encouraged new alternatives, such as the use of immunomodulatory nutrients. The objective of this study was to determine the serum levels of vitamin A (retinol), vitamin D (25(OH)VD3), and zinc (Zn) in dogs with CanL and the effect of in vitro supplementation with the respective active forms ATRA, 1,25(OH)2VD3, and SZn on spleen leukocyte cultures. Serum retinol, 25(OH)VD3, and Zn were determined by HPLC, ELISA, and ICP-MS, respectively. Spleen leukocyte cultures were used for the detection of NO and ROS by flow cytometry; the IFN-γ, TNF-α, and IL-10 levels were determined by ELISA; and the parasite load was determined by microscopy. We detected low serum levels of retinol and Zn and high levels of 25(OH)VD3 in the CanL group. The in vitro supplementation of CanL spleen leukocytes with ATRA, 1,25(OH)2VD3, and SZn, in addition to a soluble leishmania antigen (SLA) treatment, increased the NO and ROS levels, while the treatments with only ATRA and SZn increased the TNF-a levels. Increased IL-10 and IFN-g levels were observed with the addition of SLA to the medium, although the addition of the three nutrients led to a reduction of the IL-10 levels, and the addition of 1,25(OH)2VD3 and SZn led to a reduction of IFN-g. A supplementation with 1,25(OH)2VD3 and SZn reduced the parasite load but only in the absence of SLA. We suggest that the nutrients we tested are involved in the leishmanicidal mechanism, showing a potential for investigation in future studies.
Keywords: Leishmania spp.; all-trans retinoic acid; retinol; vitamin D3; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Functions of 1,25-dihydroxy vitamin D3, vitamin D3 receptor and interleukin-22 involved in pathogenesis of gout arthritis through altering metabolic pattern and inflammatory responses.PeerJ. 2021 Dec 1;9:e12585. doi: 10.7717/peerj.12585. eCollection 2021. PeerJ. 2021. PMID: 34917427 Free PMC article.
-
miR-148a regulation interferes in inflammatory cytokine and parasitic load in canine leishmaniasis.PLoS Negl Trop Dis. 2023 Jan 31;17(1):e0011039. doi: 10.1371/journal.pntd.0011039. eCollection 2023 Jan. PLoS Negl Trop Dis. 2023. PMID: 36719867 Free PMC article.
-
miRNA-21 regulates CD69 and IL-10 expression in canine leishmaniasis.PLoS One. 2022 Mar 24;17(3):e0265192. doi: 10.1371/journal.pone.0265192. eCollection 2022. PLoS One. 2022. PMID: 35324917 Free PMC article.
-
Cutaneous immune mechanisms in canine leishmaniosis due to Leishmania infantum.Vet Immunol Immunopathol. 2015 Feb 15;163(3-4):94-102. doi: 10.1016/j.vetimm.2014.11.011. Epub 2014 Nov 20. Vet Immunol Immunopathol. 2015. PMID: 25555497 Review.
-
Current status on prevention and treatment of canine leishmaniasis.Vet Parasitol. 2016 Aug 30;227:98-114. doi: 10.1016/j.vetpar.2016.07.011. Epub 2016 Jul 12. Vet Parasitol. 2016. PMID: 27523945 Review.
Cited by
-
Pet Wellness and Vitamin A: A Narrative Overview.Animals (Basel). 2024 Mar 25;14(7):1000. doi: 10.3390/ani14071000. Animals (Basel). 2024. PMID: 38612239 Free PMC article. Review.
-
Serum 25-hydroxyvitamin D and C-reactive protein and plasma von Willebrand concentrations in 23 dogs with chronic hepatopathies.J Vet Intern Med. 2022 May;36(3):966-975. doi: 10.1111/jvim.16424. Epub 2022 Apr 14. J Vet Intern Med. 2022. PMID: 35420222 Free PMC article.
-
Enzyme-derived deer velvet extract activate the immune response in cyclophosphamide-induced immunosuppressive mice.Food Sci Biotechnol. 2023 Feb 21;32(10):1435-1444. doi: 10.1007/s10068-023-01275-4. eCollection 2023 Sep. Food Sci Biotechnol. 2023. PMID: 37457410 Free PMC article.
References
-
- World Health Organization Magnitude of the Problem. Leishmaniasis. 2019. [(accessed on 26 November 2019)]. Available online: https://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/
-
- Santos W.L.C., Jesus E.E., Paranhos-Silva M., Pereira A.M., Santos J.C., Baleeiro C.O., Nascimento E.G., Moreira E.D., Oliveira G.G.S., Pontes de Carvalho L.C. Associations among immunological, parasitological and clinical parameters in canine visceral leishmaniasis: Emaciation, spleen parasitism, specific antibodies and leishmanin skin test reaction. Vet. Immunol. Immunophatol. 2008;123:251–259. doi: 10.1016/j.vetimm.2008.02.004. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

