Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758)
- PMID: 34573587
- PMCID: PMC8467327
- DOI: 10.3390/ani11092621
Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758)
Abstract
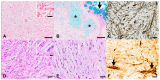
Skin spindle cell tumors (SSTs) frequently occur in fishes, with peripheral nerve sheath tumors (PNSTs) being the most commonly reported neoplasms in goldfish. However, distinguishing PNSTs from other SCTs is not always possible when relying exclusively on routine cytological and histopathological findings. Therefore, the aim of this study is to characterize six skin nodules, resembling atypical neurofibromas in humans, found in six cohabiting goldfish (Carassius auratus), and to determine a minimal subset of special stains required to correctly identify PNSTs in this species. Routine cytology and histopathology were indicative of an SCT with nuclear atypia in all cases, with randomly distributed areas of hypercellularity and loss of neurofibroma architecture. Muscular and fibroblastic tumors were excluded using Azan trichrome staining. Alcian blue and Gomori's reticulin stains revealed the presence of intratumoral areas of glycosaminoglycans or mucins and basement membrane fragments, respectively. PAS and PAS-diastase stains confirmed the latter finding and revealed intra- and extracellular glycogen granules. Immunohistochemistry displayed multifocal, randomly distributed aggregates of neoplastic cells positive for S100 protein and CNPase, intermingled with phosphorylated and non-phosphorylated neurofilament-positive axons. Collectively, these findings are consistent with a PNST resembling atypical neurofibroma in humans, an entity not previously reported in goldfish, and suggest that Azan trichrome staining, reticulin staining, and immunohistochemistry for S100 protein and CNPase represent a useful set of special stains to identify and characterize PNSTs in this species.
Keywords: Azan trichrome stain; CNPase; S100; reticulin stain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Immunohistochemistry for 2',3'-cyclic nucleotide-3'-phosphohydrolase in 63 bovine peripheral nerve sheath tumors.Vet Pathol. 2011 Jul;48(4):796-802. doi: 10.1177/0300985810388521. Epub 2010 Dec 1. Vet Pathol. 2011. PMID: 21123863
-
The controversial nosology of benign nerve sheath tumors: neurofilament protein staining demonstrates intratumoral axons in many sporadic schwannomas.Am J Surg Pathol. 2007 Sep;31(9):1363-70. doi: 10.1097/PAS.0b013e318031bc0c. Am J Surg Pathol. 2007. PMID: 17721192
-
Intrathoracic peripheral nerve sheath tumors-a clinicopathological study of 75 cases.Hum Pathol. 2015 Mar;46(3):419-25. doi: 10.1016/j.humpath.2014.11.017. Epub 2014 Dec 10. Hum Pathol. 2015. PMID: 25595633
-
Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature.BMC Cancer. 2017 May 19;17(1):349. doi: 10.1186/s12885-017-3350-1. BMC Cancer. 2017. PMID: 28526004 Free PMC article. Review.
-
Peripheral Nerve Sheath Tumors of Head and Neck: Imaging-Based Review of World Health Organization Classification.J Comput Assist Tomogr. 2020 Nov/Dec;44(6):928-940. doi: 10.1097/RCT.0000000000001109. J Comput Assist Tomogr. 2020. PMID: 33196600 Review.
Cited by
-
Retrospective Study of the Prevalence, Histopathology, Therapy, and Survival Time of Neoplastic Disease in Fish.Animals (Basel). 2024 Jan 31;14(3):464. doi: 10.3390/ani14030464. Animals (Basel). 2024. PMID: 38338107 Free PMC article.
-
Persistent Infection of a Canine Histiocytic Sarcoma Cell Line with Attenuated Canine Distemper Virus Expressing Vasostatin or Granulocyte-Macrophage Colony-Stimulating Factor.Int J Mol Sci. 2022 May 31;23(11):6156. doi: 10.3390/ijms23116156. Int J Mol Sci. 2022. PMID: 35682834 Free PMC article.
-
Cytology of a seminoma in a koi (Cyprinus carpio): a rapid diagnostic tool.Vet Res Commun. 2024 Aug;48(4):2589-2593. doi: 10.1007/s11259-024-10391-3. Epub 2024 May 21. Vet Res Commun. 2024. PMID: 38769240 Free PMC article.
-
Exploring Immunohistochemistry in Fish: Assessment of Antibody Reactivity by Western Immunoblotting.Animals (Basel). 2023 Sep 15;13(18):2934. doi: 10.3390/ani13182934. Animals (Basel). 2023. PMID: 37760333 Free PMC article.
References
-
- Meuten D.J. Tumors in Domestic Animals. 5th ed. Wiley Blackwell; Hoboken, NJ, USA: 2017. [(accessed on 5 September 2021)]. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119181200. - DOI
-
- Quesenberry K., Carpenter J. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery. Elsevier; Amsterdam, The Netherlands: 2012. [(accessed on 5 September 2021)]. Available online: https://www.elsevier.com/books/ferrets-rabbits-and-rodents/quesenberry/9....
-
- Terio K., McAloose D., Leger J. Pathology of Wildlife and Zoo Animals. 1st ed. Academic Press; Waltham, MA, USA: 2018. [(accessed on 5 September 2021)]. Available online: https://www.elsevier.com/books/pathology-of-wildlife-and-zoo-animals/ter....

