A Drive towards Thermodynamic Efficiency for Dissipative Structures in Chemical Reaction Networks
- PMID: 34573740
- PMCID: PMC8472781
- DOI: 10.3390/e23091115
A Drive towards Thermodynamic Efficiency for Dissipative Structures in Chemical Reaction Networks
Abstract
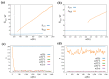
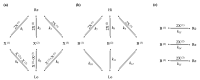
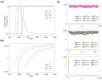
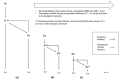
Dissipative accounts of structure formation show that the self-organisation of complex structures is thermodynamically favoured, whenever these structures dissipate free energy that could not be accessed otherwise. These structures therefore open transition channels for the state of the universe to move from a frustrated, metastable state to another metastable state of higher entropy. However, these accounts apply as well to relatively simple, dissipative systems, such as convection cells, hurricanes, candle flames, lightning strikes, or mechanical cracks, as they do to complex biological systems. Conversely, interesting computational properties-that characterize complex biological systems, such as efficient, predictive representations of environmental dynamics-can be linked to the thermodynamic efficiency of underlying physical processes. However, the potential mechanisms that underwrite the selection of dissipative structures with thermodynamically efficient subprocesses is not completely understood. We address these mechanisms by explaining how bifurcation-based, work-harvesting processes-required to sustain complex dissipative structures-might be driven towards thermodynamic efficiency. We first demonstrate a simple mechanism that leads to self-selection of efficient dissipative structures in a stochastic chemical reaction network, when the dissipated driving chemical potential difference is decreased. We then discuss how such a drive can emerge naturally in a hierarchy of self-similar dissipative structures, each feeding on the dissipative structures of a previous level, when moving away from the initial, driving disequilibrium.
Keywords: chemical reaction networks; dissipative structures; stochastic thermodynamics; thermodynamic efficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Morowitz H., Smith E. Energy flow and the organization of life. Complexity. 2007;13:51–59. doi: 10.1002/cplx.20191. - DOI
-
- Smith E., Morowitz H.J. The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere. Cambridge University Press; Cambridge, UK: 2016.
Grants and funding
LinkOut - more resources
Full Text Sources

