Blood-Based Biomarkers of Neuroinflammation in Alzheimer's Disease: A Central Role for Periphery?
- PMID: 34573867
- PMCID: PMC8464786
- DOI: 10.3390/diagnostics11091525
Blood-Based Biomarkers of Neuroinflammation in Alzheimer's Disease: A Central Role for Periphery?
Abstract
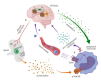
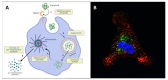
Neuroinflammation represents a central feature in the development of Alzheimer's disease (AD). The resident innate immune cells of the brain are the principal players in neuroinflammation, and their activation leads to a defensive response aimed at promoting β-amyloid (Aβ) clearance. However, it is now widely accepted that the peripheral immune system-by virtue of a dysfunctional blood-brain barrier (BBB)-is involved in the pathogenesis and progression of AD; microglial and astrocytic activation leads to the release of chemokines able to recruit peripheral immune cells into the central nervous system (CNS); at the same time, cytokines released by peripheral cells are able to cross the BBB and act upon glial cells, modifying their phenotype. To successfully fight this neurodegenerative disorder, accurate and sensitive biomarkers are required to be used for implementing an early diagnosis, monitoring the disease progression and treatment effectiveness. Interestingly, as a result of the bidirectional communication between the brain and the periphery, the blood compartment ends up reflecting several pathological changes occurring in the AD brain and can represent an accessible source for such biomarkers. In this review, we provide an overview on some of the most promising peripheral biomarkers of neuroinflammation, discussing their pathogenic role in AD.
Keywords: Alzheimer’s disease; TSPO; chemokines; cytokines; delirium; monocytes; neuroinflammation; peripheral markers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

