Clonal Evolution of Multiple Myeloma-Clinical and Diagnostic Implications
- PMID: 34573876
- PMCID: PMC8469181
- DOI: 10.3390/diagnostics11091534
Clonal Evolution of Multiple Myeloma-Clinical and Diagnostic Implications
Abstract
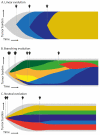
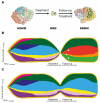
Plasma cell dyscrasias are a heterogeneous group of diseases characterized by the expansion of bone marrow plasma cells. Malignant transformation of plasma cells depends on the continuity of events resulting in a sequence of well-defined disease stages, from monoclonal gammopathy of undetermined significance (MGUS) through smoldering myeloma (SMM) to symptomatic multiple myeloma (MM). Evolution of a pre-malignant cell into a malignant cell, as well as further tumor progression, dissemination, and relapse, require development of multiple driver lesions conferring selective advantage of the dominant clone and allowing subsequent evolution under selective pressure of microenvironment and treatment. This process of natural selection facilitates tumor plasticity leading to the formation of genetically complex and heterogenous tumors that are notoriously difficult to treat. Better understanding of the mechanisms underlying tumor evolution in MM and identification of lesions driving the evolution from the premalignant clone is therefore a key to development of effective treatment and long-term disease control. Here, we review recent advances in clonal evolution patterns and genomic landscape dynamics of MM, focusing on their clinical implications.
Keywords: clonal evolution; genetic heterogeneity; multiple myeloma; tumor heterogeneity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
From MGUS to Multiple Myeloma, a Paradigm for Clonal Evolution of Premalignant Cells.Cancer Res. 2018 May 15;78(10):2449-2456. doi: 10.1158/0008-5472.CAN-17-3115. Epub 2018 Apr 27. Cancer Res. 2018. PMID: 29703720 Review.
-
Changing paradigms in diagnosis and treatment of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM).Leukemia. 2020 Dec;34(12):3111-3125. doi: 10.1038/s41375-020-01051-x. Epub 2020 Oct 12. Leukemia. 2020. PMID: 33046818 Review.
-
Biological and Clinical Implications of Clonal Heterogeneity and Clonal Evolution in Multiple Myeloma.Curr Cancer Ther Rev. 2014;10(2):70-79. doi: 10.2174/157339471002141124121404. Curr Cancer Ther Rev. 2014. PMID: 25705146 Free PMC article.
-
Diagnosis, risk stratification and management of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma.Int J Lab Hematol. 2016 May;38 Suppl 1:110-22. doi: 10.1111/ijlh.12504. Epub 2016 May 9. Int J Lab Hematol. 2016. PMID: 27161311 Review.
-
Genomics of Smoldering Multiple Myeloma: Time for Clinical Translation of Findings?Cancers (Basel). 2021 Jul 1;13(13):3319. doi: 10.3390/cancers13133319. Cancers (Basel). 2021. PMID: 34282760 Free PMC article. Review.
Cited by
-
Identification of evolutionary mechanisms of myelomatous effusion by single-cell RNA sequencing.Blood Adv. 2023 Aug 8;7(15):4148-4159. doi: 10.1182/bloodadvances.2022009477. Blood Adv. 2023. PMID: 37276129 Free PMC article.
-
Relapsed/refractory multiple myeloma: standard of care management of patients in the Gulf region.Clin Hematol Int. 2025 May 8;7(2):20-33. doi: 10.46989/001c.137860. eCollection 2025. Clin Hematol Int. 2025. PMID: 40352314 Free PMC article. Review.
-
Multiple Myeloma Insights from Single-Cell Analysis: Clonal Evolution, the Microenvironment, Therapy Evasion, and Clinical Implications.Cancers (Basel). 2025 Feb 14;17(4):653. doi: 10.3390/cancers17040653. Cancers (Basel). 2025. PMID: 40002248 Free PMC article. Review.
-
A Phase I Trial Evaluating the Addition of Lenalidomide to Patients with Relapsed/Refractory Multiple Myeloma Progressing on Ruxolitinib and Methylprednisolone.Target Oncol. 2024 May;19(3):343-357. doi: 10.1007/s11523-024-01049-w. Epub 2024 Apr 20. Target Oncol. 2024. PMID: 38643346 Clinical Trial.
-
Overview of 1q abnormalities in multiple myeloma: scientific opinions from Italian experts.Ann Hematol. 2025 Mar;104(3):1443-1458. doi: 10.1007/s00277-025-06212-5. Epub 2025 Feb 13. Ann Hematol. 2025. PMID: 39945832 Free PMC article. Review.
References
-
- Dimopoulos M.A., Moreau P., Palumbo A., Joshua D., Pour L., Hájek R., Facon T., Ludwig H., Oriol A., Goldschmidt H., et al. Carfilzomib and Dexamethasone versus Bortezomib and Dexamethasone for Patients with Relapsed or Refractory Multiple Myeloma (ENDEAVOR): A Randomised, Phase 3, Open-Label, Multicentre Study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. - DOI - PubMed
-
- Mateos M.V., Richardson P.G., Schlag R., Khuageva N.K., Dimopoulos M.A., Shpilberg O., Kropff M., Spicka I., Petrucci M.T., Palumbo A., et al. MPV Compared with MP in Previously Untreated Multiple Myeloma_Updated Follow-up in VISTA Trial. J. Clin. Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. - DOI - PubMed
-
- Miguel J.S., Weisel K., Moreau P., Lacy M., Song K., Delforge M., Karlin L., Goldschmidt H., Banos A., Oriol A., et al. Pomalidomide plus Low-Dose Dexamethasone versus High-Dose Dexamethasone Alone for Patients with Relapsed and Refractory Multiple Myeloma (MM-003): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

