Performance of Seven SARS-CoV-2 Self-Tests Based on Saliva, Anterior Nasal and Nasopharyngeal Swabs Corrected for Infectiousness in Real-Life Conditions: A Cross-Sectional Test Accuracy Study
- PMID: 34573909
- PMCID: PMC8466378
- DOI: 10.3390/diagnostics11091567
Performance of Seven SARS-CoV-2 Self-Tests Based on Saliva, Anterior Nasal and Nasopharyngeal Swabs Corrected for Infectiousness in Real-Life Conditions: A Cross-Sectional Test Accuracy Study
Abstract
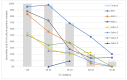
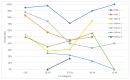
Many studies reported good performance of nasopharyngeal swab-based antigen tests for detecting SARS-CoV-2-positive individuals; however, studies independently evaluating the quality of antigen tests utilizing anterior nasal swabs or saliva swabs are still rare, although such tests are widely used for mass testing. In our study, sensitivities, specificities and predictive values of seven antigen tests for detection of SARS-CoV-2 (one using nasopharyngeal swabs, two using anterior nasal swabs and four using saliva) were evaluated. In a setting of a high-capacity testing center, nasopharyngeal swabs for quantitative PCR (qPCR) were taken and, at the same time, antigen testing was performed in accordance with manufacturers' instructions for the respective tests. In samples where qPCR and antigen tests yielded different results, virus culture was performed to evaluate the presence of the viable virus. Sensitivities and specificities of individual tests were calculated using both qPCR and qPCR corrected for viability as the reference. In addition, calculations were also performed for data categorized according to the cycle threshold and symptomatic status. The test using nasopharyngeal swabs yielded the best results (sensitivity of 80.6% relative to PCR and 91.2% when corrected for viability) while none of the remaining tests (anterior nasal swab or saliva-based tests) came even close to the WHO criteria for overall sensitivity. Hence, we advise caution when using antigen tests with alternative sampling methods without independent validation.
Keywords: COVID-19; SARS-CoV-2; anterior nasal swab; evaluation; qPCR; rapid antigen tests; saliva; virus culture.
Conflict of interest statement
Tests were provided by the distributors of the tests who also contributed equally to the costs of testing. However, the funders had no role in the design of the study; in the collection, analyses and interpretation of data; or in the writing of the article. However, under the terms of the agreements with the funders, without which this study could not be performed at all, we are not allowed to publish the test names or manufacturers.
Figures
Similar articles
-
An Agreement of Antigen Tests on Oral Pharyngeal Swabs or Less Invasive Testing With Reverse Transcription Polymerase Chain Reaction for Detecting SARS-CoV-2 in Adults: Protocol for a Prospective Nationwide Observational Study.JMIR Res Protoc. 2022 May 4;11(5):e35706. doi: 10.2196/35706. JMIR Res Protoc. 2022. PMID: 35394449 Free PMC article.
-
Performance of anterior nares and tongue swabs for nucleic acid, Nucleocapsid, and Spike antigen testing for detecting SARS-CoV-2 against nasopharyngeal PCR and viral culture.Int J Infect Dis. 2022 Apr;117:287-294. doi: 10.1016/j.ijid.2022.02.009. Epub 2022 Feb 9. Int J Infect Dis. 2022. PMID: 35149246 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
Diagnostic Performance Assessment of Saliva RT-PCR and Nasopharyngeal Antigen for the Detection of SARS-CoV-2 in Peru.Microbiol Spectr. 2022 Aug 31;10(4):e0086122. doi: 10.1128/spectrum.00861-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867471 Free PMC article.
-
Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and meta-analysis.PLoS One. 2021 Jul 20;16(7):e0254559. doi: 10.1371/journal.pone.0254559. eCollection 2021. PLoS One. 2021. PMID: 34283845 Free PMC article. Review.
Cited by
-
Economic evaluation of COVID-19 rapid antigen screening programs in the workplace.BMC Med. 2022 Nov 23;20(1):452. doi: 10.1186/s12916-022-02641-5. BMC Med. 2022. PMID: 36424587 Free PMC article.
-
Self-testing for SARS-CoV-2 in São Paulo, Brazil: results of a population-based values and attitudes survey.BMC Infect Dis. 2022 Sep 2;22(1):720. doi: 10.1186/s12879-022-07706-7. BMC Infect Dis. 2022. PMID: 36056299 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Comparison of Nasal Swabs, Nasopharyngeal Swabs, and Saliva Samples for the Detection of SARS-CoV-2 and other Respiratory Virus Infections.Ann Lab Med. 2023 Sep 1;43(5):434-442. doi: 10.3343/alm.2023.43.5.434. Epub 2023 Apr 21. Ann Lab Med. 2023. PMID: 37080744 Free PMC article.
-
Head-to-head comparison of the accuracy of saliva and nasal rapid antigen SARS-CoV-2 self-testing: cross-sectional study.BMC Med. 2022 Oct 24;20(1):406. doi: 10.1186/s12916-022-02603-x. BMC Med. 2022. PMID: 36280827 Free PMC article.
References
-
- ECDC . Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. ECDC; Solna Municipality, Sweden: 2020.
-
- Brümmer L.E., Katzenschlager S., Gaeddert M., Erdmann C., Schmitz S., Bota M., Grilli M., Larmann J., Weigand M.A., Pollock N.R., et al. The accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. medRxiv. 2021 doi: 10.1101/2021.02.26.21252546. - DOI - PMC - PubMed
-
- Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., Emperador D., Takwoingi Y., Cunningham J., Beese S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020;8:CD013705. doi: 10.1002/14651858.cd013705. - DOI - PMC - PubMed
-
- ECDC ECDC Technical Report: Considerations Onthe Use of Self-Testsfor COVID-19 in the EU/EEA. [(accessed on 6 June 2021)]. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Considerations-....
LinkOut - more resources
Full Text Sources
Miscellaneous

