Phase II Metabolism of Asarone Isomers In Vitro and in Humans Using HPLC-MS/MS and HPLC-qToF/MS
- PMID: 34574142
- PMCID: PMC8467817
- DOI: 10.3390/foods10092032
Phase II Metabolism of Asarone Isomers In Vitro and in Humans Using HPLC-MS/MS and HPLC-qToF/MS
Abstract
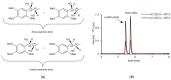
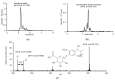
(1) Background: Metabolism data of asarone isomers, in particular phase II, in vitro and in humans is limited so far. For the first time, phase II metabolites of asarone isomers were characterized and human kinetic as well as excretion data after oral intake of asarone-containing tea infusion was determined. (2) Methods: A high pressure liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (HPLC-qTOF-MS) approach was used to identify phase II metabolites using liver microsomes of different species and in human urine samples. For quantitation of the respective glucuronides, a beta-glucuronidase treatment was performed prior to analysis via high pressure liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). (3) Results: Ingested beta-asarone and erythro and threo-asarone diols were excreted as diols and respective diol glucuronide conjugates within 24 h. An excretion rate about 42% was estimated. O-Demethylation of beta-asarone was also indicated as a human metabolic pathway because a corresponding glucuronic acid conjugate was suggested. (4) Conclusions: Already reported O-demethylation and epoxide-derived diols formation in phase I metabolism of beta-asarone in vitro was verified in humans and glucuronidation was characterized as main conjugation reaction. The excretion rate of 42% as erythro and threo-asarone diols and respective asarone diol glucuronides suggests that epoxide formation is a key step in beta-asarone metabolism, but further, as yet unknown metabolites should also be taken into consideration.
Keywords: asarone glucuronides; asarone isomers; human study; metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Varshney V.K., Song B.H., Ginwal H.S., Mittal N. High Levels of diversity in the phytochemistry, ploidy and genetics of the medicinal plant Acorus calamus L. J. Med. Aromat. Plants. 2015;2015:1–9. doi: 10.4172/2167-0412.S1-002. - DOI
-
- Scientific Committee on Food. European Commission Opinion of the Scientific Committee on Food on the Presence of Beta-asarone in Flavourings and Other Food Ingredients with Flavouring Properties (SCF/CS/FLAV/FLAVOUR/9 ADD1 Final) 2002. [(accessed on 27 August 2021)]. Available online: https://ec.europa.eu/food/system/files/2016-10/fs_food-improvement-agent....
Grants and funding
LinkOut - more resources
Full Text Sources

