Remdesivir in Severe COVID-19 and Non-Invasive Ventilation: A Real-Life Experience
- PMID: 34574882
- PMCID: PMC8464871
- DOI: 10.3390/healthcare9091108
Remdesivir in Severe COVID-19 and Non-Invasive Ventilation: A Real-Life Experience
Abstract
Background: Antiviral treatment is a hot topic regarding therapy for COVID-19. Several antiviral drugs have been tested in the months since the pandemic began. Yet only Remdesivir obtained approval after first trials. The best time to administer Remdesivir is still a matter for discussion and this could also depend upon the severity of lung damage and the staging of the infection.
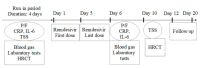
Methods: We performed a real-life study of patients hospitalized forCOVID-19 and receiving non-invasive ventilation (NIV). In this single-center study, a 5 day course of Remdesivir was administered as compassionate use. Further therapeutic supports included antibiotics, low molecular weight heparin and steroids. Data collection included clinical signs and symptoms, gas exchange, laboratory markers of inflammation, and radiological findings. Major outcomes were de-escalation of oxygen-support requirements, clinical improvement defined by weaning from ventilation to oxygen therapy or discharge, and mortality. Adverse drug reactions were also recorded. All data were collected during hospitalization and during a 20-day follow up after treatment.
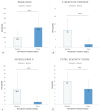
Results: 51 patients were enrolled. A global clinical improvement was recorded in 22 patients (43%) at 12 days, and 36 (71%) at 20 days; in particular, at 12 days, 27 patients (53%) also had a de-escalation of oxygen-support class from a therapeutic point of view. Remdesivir use was associated with a lower hazard ratio for clinical improvement in the elderly (older than 70 years) and in subjects with more extensive lung involvement (total severity score at HRCT of more than 14). The 20-day mortality was 13%.
Conclusions: Results demonstrated that Remdesivir is associated with an improvement in clinical, laboratory and radiological parameters in patients with severe COVID-19 and showed an overall mortality of 13%. We conclude that, in this cohort, Remdesivir was a beneficial add-on therapy for severe COVID-19, especially in adults with moderate lung involvement at HRCT.
Keywords: ARDS; CPAP; NIV; SARS-CoV2; antiviral.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 17 May 2021)]. Available online: https://covid19.who.int/
LinkOut - more resources
Full Text Sources
Miscellaneous

