Weekly Semaglutide vs. Liraglutide Efficacy Profile: A Network Meta-Analysis
- PMID: 34574899
- PMCID: PMC8466858
- DOI: 10.3390/healthcare9091125
Weekly Semaglutide vs. Liraglutide Efficacy Profile: A Network Meta-Analysis
Abstract
Introduction: Glucagon-like peptide 1 receptor agonist (GLP-1 RA) is a class of hypoglycemic medications. Semaglutide once-weekly (QW) and liraglutide once-daily (OD) significantly improved glycemic control compared to placebo. To date, no long-term phase III trials directly comparing semaglutide and liraglutide are available. This network meta-analysis (NMA) aims to compare the long-term efficacy of semaglutide and liraglutide.
Methods: PubMed, Embase, and Cochrane Library were searched from inception until June 2019 to identify relevant articles. Nine long-term randomized controlled trials comparing once-weekly semaglutide or liraglutide with placebo or other active comparisons were identified. The outcomes of interest were changes in HbA1c and weight after 52 weeks. A Bayesian framework and NMA were used for data synthesis. This is a sub-study of the protocol registered in PROSPERO (number CRD42018091598).
Results: The data showed significant superiority in HbA1c reduction of semaglutide 1 mg QW over liraglutide 1.2 and 1.8 mg with a treatment difference of 0.47% and 0.3%, respectively. Semaglutide 0.5 mg QW was found to be significantly superior to liraglutide 1.2 mg in HbA1c reduction with a treatment difference of 0.17%. Regarding weight reduction analysis, semaglutide 0.5 and 1 mg QW were significantly associated with a greater reduction than liraglutide 0.6 mg with a treatment difference of 2.42 and 3.06 kg, respectively. However, no significant reduction was found in comparison to liraglutide 1.2 and 1.8 mg.
Conclusions: Semaglutide improved the control of blood glucose and body weight. The capacity of long-term glycemic control and body weight control of semaglutide appears to be more effective than other GLP-1 RAs, including liraglutide. However, considering the number of included studies and potential limitations, more large-scale, head-to-head, well-designed randomized-controlled trials (RCTs) are needed to confirm these findings.
Keywords: GLP-1; GLP-1 RA; Glucagon-like peptide; HbA1c; diabetes mellitus; glycemic control; liraglutide; network meta-analysis; semaglutide; weight.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

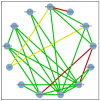
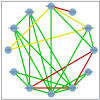


References
-
- Association A.D. Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016;39((Suppl. 1)):S4–S5. - PubMed
-
- Zoungas S., Chalmers J., Ninomiya T., Li Q., Cooper M.E., Colagiuri S., Fulcher G., De Galan B.E., Harrap S., Hamet P., et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: Evidence of glycaemic thresholds. Diabetologia. 2012;55:636–643. doi: 10.1007/s00125-011-2404-1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

