Aortic Valve Stenosis and Cardiac Amyloidosis: A Misleading Association
- PMID: 34575344
- PMCID: PMC8471197
- DOI: 10.3390/jcm10184234
Aortic Valve Stenosis and Cardiac Amyloidosis: A Misleading Association
Abstract
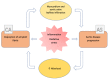
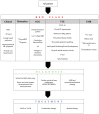
The association between aortic stenosis (AS) and cardiac amyloidosis (CA) is more frequent than expected. Albeit rare, CA, particularly the transthyretin (ATTR) form, is commonly found in elderly people. ATTR-CA is also the most prevalent form in patients with AS. These conditions share pathophysiological, clinical and imaging findings, making the diagnostic process very challenging. To date, a multiparametric evaluation is suggested in order to detect patients with both AS and CA and choose the best therapeutic option. Given the accuracy of modern non-invasive techniques (i.e., bone scintigraphy), early diagnosis of CA is possible. Flow-charts with the main CA findings which may help clinicians in the diagnostic process have been proposed. The prognostic impact of the combination of AS and CA is not fully known; however, new available specific treatments of ATTR-CA have changed the natural history of the disease and have some impact on the decision-making process for the management of AS. Hence the relevance of detecting these two conditions when simultaneously present. The specific features helping the detection of AS-CA association are discussed in this review, focusing on the shared pathophysiological characteristics and the common clinical and imaging hallmarks.
Keywords: amyloidosis; aortic valve stenosis; multimodality imaging; transthyretin amyloidosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Balciunaite G., Rimkus A., Zurauskas E., Zaremba T., Palionis D., Valeviciene N., Aidietis A., Serpytis P., Rucinskas K., Sogaard P., et al. Transthyretin cardiac amyloidosis in aortic stenosis: Prevalence, diagnostic challenges, and clinical implications. Hell J. Cardiol. HJC Hell Kardiol. Ep. 2020;61:92–98. doi: 10.1016/j.hjc.2019.10.004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

