Bleomycin Concentration in Patients' Plasma and Tumors after Electrochemotherapy. A Study from InspECT Group
- PMID: 34575400
- PMCID: PMC8469090
- DOI: 10.3390/pharmaceutics13091324
Bleomycin Concentration in Patients' Plasma and Tumors after Electrochemotherapy. A Study from InspECT Group
Abstract
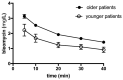
The plasma concentration profile of bleomycin in the distribution phase of patients younger than 65 years is needed to determine the suitable time interval for efficient application of electric pulses during electrochemotherapy. Additionally, bleomycin concentrations in the treated tumors for effective tumor response are not known. In this study, the pharmacokinetic profile of bleomycin in the distribution phase in 12 patients younger than 65 years was determined. In 17 patients, the intratumoral bleomycin concentration was determined before the application of electric pulses. In younger patients, the pharmacokinetics of intravenously injected bleomycin demonstrated a faster plasma clearance rate than that in patients older than 65 years. This outcome might indicate that the lowering of the standard bleomycin dose of 15,000 IU/m2 with intravenous bleomycin injection for electrochemotherapy is not recommended in younger patients. Based on the plasma concentration data gathered, a time interval for electrochemotherapy of 5-15 min after bleomycin injection was determined. The median bleomycin concentration in tumors 8 min after bleomycin injection, at the time of electroporation, was 170 ng/g. Based on collected data, the reduction of the bleomycin dose is not recommended in younger patients; however, a shortened time interval for application of electric pulses in electrochemotherapy to 5-15 min after intravenous bleomycin injection should be considered.
Keywords: bleomycin; blood samples; drug delivery; electrochemotherapy; electroporation; pharmacokinetics; tumor samples.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Campana L.G., Miklavčič D., Bertino G., Marconato R., Valpione S., Imarisio I., Dieci M.V., Granziera E., Cemazar M., Alaibac M., et al. Electrochemotherapy of superficial tumors—Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin. Oncol. 2019;46:173–191. doi: 10.1053/j.seminoncol.2019.04.002. - DOI - PubMed
-
- Bertino G., Sersa G., De Terlizzi F., Occhini A., Plaschke C.C., Groselj A., Langdon C., Grau J.J., McCaul J.A., Heuveling D., et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer. 2016;63:41–52. doi: 10.1016/j.ejca.2016.05.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

