Exploring Taxifolin Polymorphs: Insights on Hydrate and Anhydrous Forms
- PMID: 34575404
- PMCID: PMC8469002
- DOI: 10.3390/pharmaceutics13091328
Exploring Taxifolin Polymorphs: Insights on Hydrate and Anhydrous Forms
Abstract
Taxifolin, also known as dihydroquercetin, possesses several interesting biological properties. The purpose of the study was to identify polymorphs of taxifolin prepared using crystallization in different solvents. Data from X-ray powder diffraction, differential scanning calorimetry, and thermogravimetry enabled us to detect six different crystalline phases for taxifolin. Besides the already known fully hydrated phase, one partially hydrated phase, one monohydrated phase, two anhydrous polymorphs, and one probably solvated phase were obtained. The unit cell parameters were defined for three of them, while one anhydrous polymorph was fully structurally characterized by X-ray powder diffraction data. Scanning electron microscopy and hot stage microscopy were also employed to characterize the crystallized taxifolin powders. The hydrate and anhydrous forms showed remarkable stability in drastic storage conditions, and their solubility was deeply evaluated. The anhydrous form converted into the hydrate form during the equilibrium solubility study and taxifolin equilibrium solubility was about 1.2 mg/mL. The hydrate taxifolin intrinsic dissolution rate was 56.4 μg cm-2 min-1. Using Wood's apparatus, it was not possible to determine the intrinsic dissolution rate of anhydrous taxifolin that is expected to solubilize more rapidly than the hydrate form. In view of its high stability, its use can be hypothesized.
Keywords: X-ray powder diffraction; differential scanning calorimetry; equilibrium solubility; intrinsic solubility; polymorphism; taxifolin; thermogravimetry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





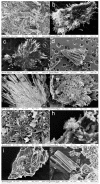
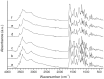

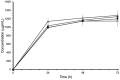

References
-
- Schlickmann F., Mota da Silva L., Boeing T., Bordignon Somensi L., de Moura Burci L., Santin J.R., Filho V.C., Faloni de Andrade S. Gastroprotective Bio-Guiding Study of Fruits from Mimusops Balata. Naunyn Schmiedebergs Arch. Pharmacol. 2015;388:1187–1200. doi: 10.1007/s00210-015-1156-8. - DOI - PubMed
-
- Kuspradini H., Mitsunaga T., Ohashi H. Antimicrobial Activity against Streptococcus Sobrinus and Glucosyltransferase Inhibitory Activity of Taxifolin and Some Flavanonol Rhamnosides from Kempas (Koompassia malaccensis) Extracts. J. Wood Sci. 2009;55:308–313. doi: 10.1007/s10086-009-1026-4. - DOI
-
- Lawrence R., Jeyakumar E., Gupta A. Antibacterial Activity of Acacia Arabica (Bark) Extract against Selected Multi Drug Resistant Pathogenic Bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2015;1:213–222.
-
- Itaya S., Igarashi K. Effects of Taxifolin on the Serum Cholesterol Level in Rats. Biosci. Biotechnol. Biochem. 1992;56:1492–1494. doi: 10.1271/bbb.56.1492. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

