Viscoelastic and Deformation Characteristics of Structurally Different Commercial Topical Systems
- PMID: 34575425
- PMCID: PMC8469870
- DOI: 10.3390/pharmaceutics13091351
Viscoelastic and Deformation Characteristics of Structurally Different Commercial Topical Systems
Abstract
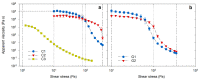
Rheological characteristics and shear response have potential implication in defining the pharmaceutical equivalence, therapeutic equivalence, and perceptive equivalence of commercial topical products. Three creams (C1 and C3 as oil-in-water and C2 as water-in-oil emulsions), and two gels (G1 and G2 carbomer-based) were characterized using the dynamic range of controlled shear in steady-state flow and oscillatory modes. All products, other than C3, met the Critical Quality Attribute criteria for high zero-shear viscosity (η0) of 2.6 × 104 to 1.5 × 105 Pa∙s and yield stress (τ0) of 55 to 277 Pa. C3 exhibited a smaller linear viscoelastic region and lower η0 (2547 Pa∙s) and τ0 (2 Pa), consistent with lotion-like behavior. All dose forms showed viscoelastic solid behavior having a storage modulus (G') higher than the loss modulus (G″) in the linear viscoelastic region. However, the transition of G' > G″ to G″ > G' during the continual strain increment was more rapid for the creams, elucidating a relatively brittle deformation, whereas these transitions in gels were more prolonged, consistent with a gradual disentanglement of the polymer network. In conclusion, these analyses not only ensure quality and stability, but also enable the microstructure to be characterized as being flexible (gels) or inelastic (creams).
Keywords: critical quality attributes (CQAs); deformation characteristics; power-law functions; rheology; topical semisolid products; viscoelastic properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ethier A., Bansal P., Baxter J., Langley N., Richardson N., Patel A.M. The role of excipients in the microstructure of Topical semisolid drug products. In: Langley N., Michniak-Kohn B., Osborne D.W., editors. The Role of Microstructure in Topical Drug Product Development. Volume 36. Springer; Cham, Switzerland: 2019. pp. 155–193.
-
- U.S. Food and Drug Administration Draft Guidance on Acyclovir. In Guidance for Industry ANDA Submissions. December 2016. [(accessed on 29 June 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_topical%20c....
LinkOut - more resources
Full Text Sources
Miscellaneous

