Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications
- PMID: 34575471
- PMCID: PMC8472364
- DOI: 10.3390/pharmaceutics13091395
Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications
Abstract
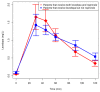
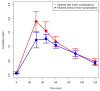
Parkinson's disease (PD) is a progressive, neurodegenerative disorder primarily affecting dopaminergic neuronal systems, with impaired motor function as a consequence. The most effective treatment for PD remains the administration of oral levodopa (LD). Long-term LD treatment is frequently associated with motor fluctuations and dyskinesias, which exert a serious impact on a patient's quality of life. The aim of our study was to determine the pharmacokinetics of LD: used as monotherapy or in combination with ropinirole, in patients with advanced PD. Furthermore, an effect of ropinirole on the pharmacokinetics of 3-OMD (a major LD metabolite) was assessed. We also investigated the correlation between the pharmacokinetic parameters of LD and 3-OMD and the occurrence of motor complications. Twenty-seven patients with idiopathic PD participated in the study. Thirteen patients received both LD and ropinirole, and fourteen administered LD monotherapy. Among 27 patients, twelve experienced fluctuations and/or dyskinesias, whereas fifteen were free of motor complications. Inter- and intra-individual variation in the LD and 3-OMD concentrations were observed. There were no significant differences in the LD and 3-OMD concentrations between the patients treated with a combined therapy of LD and ropinirole, and LD monotherapy. There were no significant differences in the LD concentrations in patients with and without motor complications; however, plasma 3-OMD levels were significantly higher in patients with motor complications. A linear one-compartment pharmacokinetic model with the first-order absorption was adopted for LD and 3-OMD. Only mean exit (residence) time for 3-OMD was significantly shorter in patients treated with ropinirole. Lag time, V/F, CL/F and tmax of LD had significantly lower values in patients with motor complications. On the other hand, AUC were significantly higher in these patients, both for LD and 3-OMD. 3-OMD Cmax was significantly higher in patients with motor complications as well. Our results showed that ropinirole does not influence LD or 3-OMD concentrations. Higher 3-OMD levels play a role in inducing motor complications during long-term levodopa therapy.
Keywords: 3-O-methyldopa; Parkinson’s disease; levodopa; motor complications; pharmacokinetics; ropinirole.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

