Preparation and In Vivo Evaluation of a Lidocaine Self-Nanoemulsifying Ointment with Glycerol Monostearate for Local Delivery
- PMID: 34575544
- PMCID: PMC8464853
- DOI: 10.3390/pharmaceutics13091468
Preparation and In Vivo Evaluation of a Lidocaine Self-Nanoemulsifying Ointment with Glycerol Monostearate for Local Delivery
Erratum in
-
Correction: Kang et al. Preparation and In Vivo Evaluation of a Lidocaine Self-Nanoemulsifying Ointment with Glycerol Monostearate for Local Delivery. Pharmaceutics 2021, 13, 1468.Pharmaceutics. 2022 Jan 10;14(1):155. doi: 10.3390/pharmaceutics14010155. Pharmaceutics. 2022. PMID: 35057114 Free PMC article.
Abstract
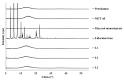
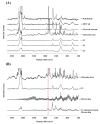
Lidocaine, a commonly used local anesthetic, has recently been developed into a number of ointment products to treat hemorrhoids. This study examined its efficient delivery to the dermis through the pharmaceutical improvement of hemorrhoid treatment ointments. We attempted to increase the amount of skin deposition of lidocaine by forming a nanoemulsion through the self-nanoemulsifying effect that occurs when glycerol monostearate (GMS) is saturated with water. Using Raman mapping, the depth of penetration of lidocaine was visualized and confirmed, and the local anesthetic effect was evaluated via an in vivo tail-flick test. Evaluation of the physicochemical properties confirmed that lidocaine was amorphous and evenly dispersed in the ointment. The in vitro dissolution test confirmed that the nanoemulsifying effect of GMS accelerated the release of the drug from the ointment. At a specific concentration of GMS, lidocaine penetrated deeper into the dermis; the in vitro permeation test showed similar results. When compared with reference product A in the tail-flick test, the L5 and L6 compounds containing GMS had a significantly higher anesthetic effect. Altogether, the self-nanoemulsifying effect of GMS accelerated the release of lidocaine from the ointment. The compound with 5% GMS, the lowest concentration that saturated the dermis, was deemed most appropriate.
Keywords: glycerol monostearate; hemorrhoids; lidocaine; local anesthetic ointment; self-nanoemulsification.
Conflict of interest statement
The authors declare no conflict of interest. H.-Y.P., S.-M.H. and S.-D.H. are full-time employees of Dong-A Pharm. Co. Ltd. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures








References
-
- Johanson J.F., Sonnenberg A.J.G. The prevalence of hemorrhoids and chronic constipation: An epidemiologic study. Gastroenterology. 1990;98:380–386. - PubMed
-
- Van Tol R.R., Kleijnen J., Watson A.J.M., Jongen J., Altomare D.F., Qvist N., Higuero T., Muris J.W.M., Breukink S.O. European society of coloproctology: Guideline for haemorrhoidal disease. Colorectal Dis. 2020;22:650–662. - PubMed
-
- Lorenc Z., Gökçe Ö. Tribenoside and lidocaine in the local treatment of hemorrhoids: An overview of clinical evidence. Eur. Rev. Med. Pharm. Sci. 2016;20:2742–2751. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

