Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment
- PMID: 34575557
- PMCID: PMC8469913
- DOI: 10.3390/pharmaceutics13091481
Mesenchymal Stromal Cells: Potential Option for COVID-19 Treatment
Abstract
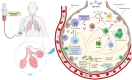
The COVID-19 pandemic has significantly impacted the way of life worldwide and continues to bring high mortality rates to at-risk groups. Patients who develop severe COVID-19 pneumonia, often complicated with ARDS, are left with limited treatment options with no targeted therapy currently available. One of the features of COVID-19 is an overaggressive immune reaction that leads to multiorgan failure. Mesenchymal stromal cell (MSC) treatment has been in development for various clinical indications for over a decade, with a safe side effect profile and promising results in preclinical and clinical trials. Therefore, the use of MSCs in COVID-19-induced respiratory failure and ARDS was a logical step in order to find a potential treatment option for the most severe patients. In this review, the main characteristics of MSCs, their proposed mechanism of action in COVID-19 treatment and the effect of this therapy in published case reports and clinical trials are discussed.
Keywords: ARDS; COVID-19; MSCs; immunomodulation; mesenchymal stromal cells.
Conflict of interest statement
The authors declare no conflict of interest. Marko Strbad, Lenart Girandon and Miomir Knežević are from Educell Ltd., Marko Strbad is from Biobanka Ltd., the companies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Zhuang W.-Z., Lin Y.-H., Su L.-J., Wu M.-S., Jeng H.-Y., Chang H.-C., Huang Y.-H., Ling T.-Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021;28:28. doi: 10.1186/s12929-021-00725-7. - DOI - PMC - PubMed
-
- Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-furman N.E., Triolo F., Kamhieh-Milz J., et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

