Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells
- PMID: 34575651
- PMCID: PMC8469227
- DOI: 10.3390/jpm11090871
Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells
Abstract
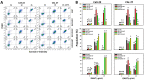
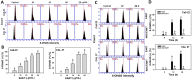
Ethyl acetate Nepenthes extract (EANT) from Nepenthes thorellii × (ventricosa × maxima) shows antiproliferation and apoptosis but not necrosis in breast cancer cells, but this has not been investigated in oral cancer cells. In the present study, EANT shows no cytotoxicity to normal oral cells but exhibits selective killing to six oral cancer cell lines. They were suppressed by pretreatment of the antioxidant inhibitor N-acetylcysteine (NAC), demonstrating that EANT-induced cell death was mediated by oxidative stress. Concerning high sensitivity to EANT, Ca9-22 and CAL 27 oral cancer cells were chosen for exploring detailed selective killing mechanisms. EANT triggers a mixture of necrosis and apoptosis as determined by annexin V/7-aminoactinmycin D analysis. Still, they show differential switches from necrosis at a low (10 μg/mL) concentration to apoptosis at high (25 μg/mL) concentration of EANT in oral cancer cells. NAC induces necrosis but suppresses annexin V-detected apoptosis in oral cancer cells. Necrostatin 1 (NEC1), a necroptosis inhibitor, moderately suppresses necrosis but induces apoptosis at 10 μg/mL EANT. In contrast, Z-VAD-FMK, a pancaspase inhibitor, slightly causes necrosis but suppresses apoptosis at 10 μg/mL EANT. Furthermore, the flow cytometry-detected pancaspase activity is dose-responsively increased but is suppressed by NAC and ZVAD, although not for NEC1 in oral cancer cells. EANT causes several oxidative stress events such as reactive oxygen species, mitochondrial superoxide, and mitochondrial membrane depolarization. In response to oxidative stresses, the mRNA for antioxidant signaling, such as nuclear factor erythroid 2-like 2 (NFE2L2), catalase (CAT), heme oxygenase 1 (HMOX1), and thioredoxin (TXN), are overexpressed in oral cancer cells. Moreover, EANT also triggers DNA damage, as detected by γH2AX and 8-oxo-2'-deoxyguanosine adducts. The dependence of oxidative stress is validated by the evidence that NAC pretreatment reverts the changes of cellular and mitochondrial stress and DNA damage. Therefore, EANT exhibits antiproliferation involving an oxidative stress-dependent necrosis/apoptosis switch and DNA damage in oral cancer cells.
Keywords: Nepenthes; apoptosis; necrosis; oral cancer; oxidative stress; preferential killing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Chen X., Wang Y., Ma N., Tian J., Shao Y., Zhu B., Wong Y.K., Liang Z., Zou C., Wang J. Target identification of natural medicine with chemical proteomics approach: Probe synthesis, target fishing and protein identification. Signal Transduct. Target. Ther. 2020;5:72. doi: 10.1038/s41392-020-0186-y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

