Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay
- PMID: 34575834
- PMCID: PMC8468575
- DOI: 10.3390/ijms22189670
Pharmaceuticals Removal by Adsorption with Montmorillonite Nanoclay
Abstract
The problem of purifying domestic and hospital wastewater from pharmaceutical compounds is becoming more and more urgent every year, because of the continuous accumulation of chemical pollutants in the environment and the limited availability of freshwater resources. Clay adsorbents have been repeatedly proposed as adsorbents for treatment purposes, but natural clays are hydrophilic and can be inefficient for catching hydrophobic pharmaceuticals. In this paper, a comparison of adsorption properties of pristine montmorillonite (MMT) and montmorillonite modified with stearyl trimethyl ammonium (hydrophobic MMT-STA) towards carbamazepine, ibuprofen, and paracetamol pharmaceuticals was performed. The efficiency of adsorption was investigated under varying solution pH, temperature, contact time, initial concentration of pharmaceuticals, and adsorbate/adsorbent mass ratio. MMT-STA was better than pristine MMT at removing all the pharmaceuticals studied. The adsorption capacity of hydrophobic montmorillonite to pharmaceuticals decreased in the following order: carbamazepine (97%) > ibuprofen (95%) > paracetamol (63-67%). Adsorption isotherms were best described by Freundlich model. Within the pharmaceutical concentration range of 10-50 µg/mL, the most optimal mass ratio of adsorbates to adsorbents was 1:300, pH 6, and a temperature of 25 °C. Thus, MMT-STA could be used as an efficient adsorbent for deconta×ating water of carbamazepine, ibuprofen, and paracetamol.
Keywords: adsorption; clay minerals; pharmaceuticals; treatment effectiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
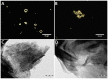



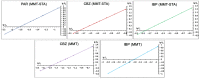


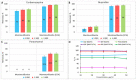
References
-
- Hai F.I., Yang S., Asif M.B., Sencadas V., Shawkat S., Sanderson-Smith M., Gorman J., Xu Z.Q., Yamamoto K. Carbamazepine as a possible anthropogenic marker in water: Occurrences, toxicological effects, regulations and removal by wastewater treatment technologies. Water. 2018;2:107. doi: 10.3390/w10020107. - DOI
-
- Macías-García A., García-Sanz-Calcedo J., Carrasco-Amador J.P., Segura-Cruz R. Adsorption of paracetamol in hospital wastewater through activated carbon filters. Sustainability. 2019;11:2672. doi: 10.3390/su11092672. - DOI
-
- Caban M., Stepnowski P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021;19:3115–3138. doi: 10.1007/s10311-021-01194-y. - DOI
-
- Lewandowski J., Meinikmann K., Krause S. Groundwater–surface water interactions: Recent advances and interdisciplinary challenges. Water. 2020;12:296. doi: 10.3390/w12010296. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

