Behavioural Functions and Cerebral Blood Flow in a P301S Tauopathy Mouse Model: A Time-Course Study
- PMID: 34575885
- PMCID: PMC8468775
- DOI: 10.3390/ijms22189727
Behavioural Functions and Cerebral Blood Flow in a P301S Tauopathy Mouse Model: A Time-Course Study
Abstract
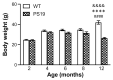
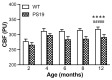
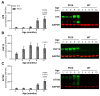
Tauopathies refer to a group of neurodegenerative diseases with intracellular accumulation of hyperphosphorylated and aggregated microtubule-associated protein tau (MAPT) in neurons and glial cells. PS19 mice bearing the MAPT P301S mutation have been used to mimic human frontotemporal lobar degeneration. The present study was designed to systematically investigate how behavioural functions, resting cerebral blood flow (CBF) and tau pathology change in PS19 mice at 2, 4, 6, 8 and 12 months of age in a single study under one experimental condition, allowing for the cumulative assessment of age- and genotype-dependent changes. PS19 mice displayed hyperactivity and reduced anxiety levels with age, early and persistent spatial working memory deficits and reduced resting neocortical CBF. Immunoblotting and immunohistochemistry revealed age-related increases in phosphorylated tau in the brain of PS19 mice. In conclusion, the present study, for the first time, cumulatively demonstrated the time-course of changes in behavioural functions, resting CBF and tau pathology in a P301S tauopathy mouse model through their developmental span. This information provides further evidence for the utility of this model to study neurodegenerative events associated with tauopathy and tau dysfunction.
Keywords: PS19 mice; anxiety; cerebral blood flow; hippocampus; hyperactivity; spatial learning and memory; tau; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
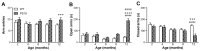




References
-
- López-González I., Aso E., Carmona M., Armand-Ugon M., Blanco R., Naudí A., Cabré R., Portero-Otin M., Pamplona R., Ferrer I. Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in tau P301S transgenic mice as a model of frontotemporal lobar degeneration-tau. J. Neuropathol. Exp. Neurol. 2015;74:975–999. doi: 10.1097/NEN.0000000000000241. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

