Novel Coumarin-Thiadiazole Hybrids and Their Cu(II) and Zn(II) Complexes as Potential Antimicrobial Agents and Acetylcholinesterase Inhibitors
- PMID: 34575894
- PMCID: PMC8471537
- DOI: 10.3390/ijms22189709
Novel Coumarin-Thiadiazole Hybrids and Their Cu(II) and Zn(II) Complexes as Potential Antimicrobial Agents and Acetylcholinesterase Inhibitors
Abstract
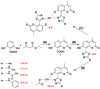
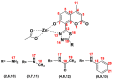
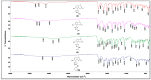
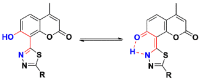
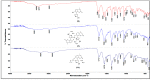
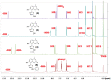
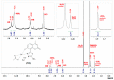
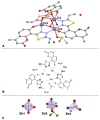
A series of coumarin-thiadiazole hybrids and their corresponding Cu(II) and Zn(II) complexes were synthesized and characterized with the use of spectroscopic techniques. The results obtained indicate that all the coumarin-thiadiazole hybrids act as bidentate chelators of Cu(II) and Zn(II) ions. The complexes isolated differ in their ligand:metal ratio depending on the central metal. In most cases, the Zn(II) complexes are characteristic of a 1:1 ligand:metal ratio, while in the Cu(II) complexes the ligand:metal ratio is 2:1. All compounds were tested as potential antibacterial agents against Gram-positive (Staphylococcus aureus, Staphylococcus epidermidis) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacterial strains demonstrating activities notably lower than commercially available antibiotics. The more promising results were obtained from the assessment of antineurodegenerative potency as all compounds showed moderate acetylcholinesterase (AChE) inhibition activity.
Keywords: acetylcholinesterase inhibitors; antibacterial activity; antimicrobial; complexes; coumarin; hybrids; neurodegeneration; thiadiazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ioannis F., Dimitra H.-L. Hybrids of Coumarin Derivatives as Potent and Multifunctional Bioactive Agents: A Review. Med. Chem. 2020;16:272–306. - PubMed
-
- Patil P.O., Bari S.B., Firke S.D., Deshmukh P.K., Donda S.T., Patil D.A. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer’s disease. Bioorg. Med. Chem. 2013;21:2434–2450. doi: 10.1016/j.bmc.2013.02.017. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

