Unveiling the Metabolic Effects of Glycomacropeptide
- PMID: 34575895
- PMCID: PMC8470927
- DOI: 10.3390/ijms22189731
Unveiling the Metabolic Effects of Glycomacropeptide
Abstract
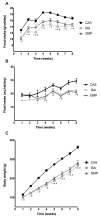
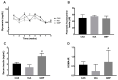
For many years, the main nitrogen source for patients with phenylketonuria (PKU) was phenylalanine-free amino acid supplements. Recently, casein glycomacropeptide (GMP) supplements have been prescribed due to its functional and sensorial properties. Nevertheless, many doubts still persist about the metabolic effects of GMP compared to free amino acids (fAA) and intact proteins such as casein (CAS). We endeavour to compare, in rats, the metabolic effects of different nitrogen sources. Twenty-four male Wistar rats were fed equal energy density diets plus CAS (control, n = 8), fAA (n = 8) or GMP (n = 8) for 8 weeks. Food, liquid intake and body weight were measured weekly. Blood biochemical parameters and markers of glycidic metabolism were assessed. Glucagon-like peptide-1 (GLP-1) was analysed by ELISA and immunohistochemistry. Food intake was higher in rats fed CAS compared to fAA or GMP throughout the treatment period. Fluid intake was similar between rats fed fAA and GMP. Body weight was systematically lower in rats fed fAA and GMP compared to those fed CAS, and still, from week 4 onwards, there were differences between fAA and GMP. None of the treatments appeared to induce consistent changes in glycaemia, while insulin levels were significantly higher in GMP. Likewise, the production of GLP-1 was higher in rats fed GMP when compared to fAA. Decreased urea, total protein and triglycerides were seen both in fAA and GMP related to CAS. GMP also reduced albumin and triglycerides in comparison to CAS and fAA, respectively. The chronic consumption of the diets triggers different metabolic responses which may provide clues to further study potential underlying mechanisms.
Keywords: GLP-1; amino acids; glycomacropeptide; intact protein; metabolism; phenylketonuria.
Conflict of interest statement
J.C.R. is member of the European Nutrition Expert Panel (BioMarin) and of the advisory boards of Applied Pharma Research and Nutricia. He has received speaker’s fees from Applied Pharma Research, Merck Serono, BioMarin, Nutricia, Vitaflo, Cambrooke, PIAM, and Lifediet.
Figures


References
-
- Van Wegberg A.M.J., MacDonald A., Ahring K., Belanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Gizewska M., et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. - DOI - PMC - PubMed
-
- Giarratana N., Gallina G., Panzeri V., Frangi A., Canobbio A., Reiner G. A new Phe-free protein substitute engineered to allow a physiological absorption of free amino acids for phenylketonuria. J. Inborn Errors Metab. Screen. 2018;6:1–9. doi: 10.1177/2326409818783780. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

