Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases
- PMID: 34575908
- PMCID: PMC8468440
- DOI: 10.3390/ijms22189744
Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases
Abstract
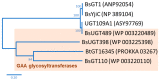
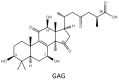
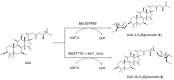
Ganoderma lucidum is a medicinal fungus abundant in triterpenoids, its primary bioactive components. Although numerous Ganoderma triterpenoids have already been identified, rare Ganoderma triterpenoid saponins were recently discovered. To create novel Ganoderma saponins, ganoderic acid G (GAG) was selected for biotransformation using four Bacillus glycosyltransferases (GTs) including BtGT_16345 from the Bacillus thuringiensis GA A07 strain and three GTs (BsGT110, BsUGT398, and BsUGT489) from the Bacillus subtilis ATCC 6633 strain. The results showed that BsUGT489 catalyzed the glycosylation of GAG to GAG-3-o-β-glucoside, while BsGT110 catalyzed the glycosylation of GAG to GAG-26-o-β-glucoside, which showed 54-fold and 97-fold greater aqueous solubility than that of GAG, respectively. To our knowledge, these two GAG saponins are new compounds. The glycosylation specificity of the four Bacillus GTs highlights the possibility of novel Ganoderma triterpenoid saponin production in the future.
Keywords: Bacillus; Ganoderma lucidum; glycosyltransferase; saponin; triterpenoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

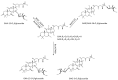
Similar articles
-
Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases.Biomolecules. 2025 May 1;15(5):655. doi: 10.3390/biom15050655. Biomolecules. 2025. PMID: 40427548 Free PMC article. Review.
-
A New Triterpenoid Glucoside from a Novel Acidic Glycosylation of Ganoderic Acid A via Recombinant Glycosyltransferase of Bacillus subtilis.Molecules. 2019 Sep 24;24(19):3457. doi: 10.3390/molecules24193457. Molecules. 2019. PMID: 31554155 Free PMC article.
-
Production of a new triterpenoid disaccharide saponin from sequential glycosylation of ganoderic acid A by 2 Bacillus glycosyltransferases.Biosci Biotechnol Biochem. 2021 Feb 24;85(3):687-690. doi: 10.1093/bbb/zbaa055. Biosci Biotechnol Biochem. 2021. PMID: 33580686
-
A Genome-Centric Approach Reveals a Novel Glycosyltransferase from the GA A07 Strain of Bacillus thuringiensis Responsible for Catalyzing 15-O-Glycosylation of Ganoderic Acid A.Int J Mol Sci. 2019 Oct 20;20(20):5192. doi: 10.3390/ijms20205192. Int J Mol Sci. 2019. PMID: 31635144 Free PMC article.
-
P450s and UGTs: Key Players in the Structural Diversity of Triterpenoid Saponins.Plant Cell Physiol. 2015 Aug;56(8):1463-71. doi: 10.1093/pcp/pcv062. Epub 2015 May 6. Plant Cell Physiol. 2015. PMID: 25951908 Free PMC article. Review.
Cited by
-
Fabrication of Mastic Gum Resin Tethered Phospholipid Nanocarriers for the Evaluation and Enhancement of Anti-inflammatory and Anti-bacterial Effects.Curr Pharm Des. 2025;31(23):1866-1876. doi: 10.2174/0113816128353794241225083428. Curr Pharm Des. 2025. PMID: 39878118
-
Enzymatic Glycosylation of Ganoderma Terpenoid via Bacterial Glycosyltransferases and Glycoside Hydrolases.Biomolecules. 2025 May 1;15(5):655. doi: 10.3390/biom15050655. Biomolecules. 2025. PMID: 40427548 Free PMC article. Review.
-
Novel Glycosylation by Amylosucrase to Produce Glycoside Anomers.Biology (Basel). 2022 May 27;11(6):822. doi: 10.3390/biology11060822. Biology (Basel). 2022. PMID: 35741343 Free PMC article.
-
Biotransformation of Natural Products and Phytochemicals: Metabolites, Their Preparation, and Properties.Int J Mol Sci. 2023 Apr 28;24(9):8030. doi: 10.3390/ijms24098030. Int J Mol Sci. 2023. PMID: 37175731 Free PMC article.
References
-
- Yang Y., Zhang H., Zuo J., Gong X., Yi F., Zhu W., Li L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019;3:6. doi: 10.1186/s41702-019-0044-0. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

