Identification of Abundant and Functional dodecaRNAs (doRNAs) Derived from Ribosomal RNA
- PMID: 34575920
- PMCID: PMC8467515
- DOI: 10.3390/ijms22189757
Identification of Abundant and Functional dodecaRNAs (doRNAs) Derived from Ribosomal RNA
Abstract
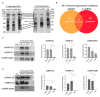
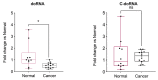
Using a modified RNA-sequencing (RNA-seq) approach, we discovered a new family of unusually short RNAs mapping to ribosomal RNA 5.8S, which we named dodecaRNAs (doRNAs), according to the number of core nucleotides (12 nt) their members contain. Using a new quantitative detection method that we developed, we confirmed our RNA-seq data and determined that the minimal core doRNA sequence and its 13-nt variant C-doRNA (doRNA with a 5' Cytosine) are the two most abundant doRNAs, which, together, may outnumber microRNAs. The C-doRNA/doRNA ratio is stable within species but differed between species. doRNA and C-doRNA are mainly cytoplasmic and interact with heterogeneous nuclear ribonucleoproteins (hnRNP) A0, A1 and A2B1, but not Argonaute 2. Reporter gene activity assays suggest that C-doRNA may function as a regulator of Annexin II receptor (AXIIR) expression. doRNAs are differentially expressed in prostate cancer cells/tissues and may control cell migration. These findings suggest that unusually short RNAs may be more abundant and important than previously thought.
Keywords: 5.8S rRNA; RNA sequencing; RT-qPCR; non-coding RNA; small RNA.
Conflict of interest statement
The authors declare no competing interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

