In Silico Study, Physicochemical, and In Vitro Lipase Inhibitory Activity of α, β-Amyrenone Inclusion Complexes with Cyclodextrins
- PMID: 34576044
- PMCID: PMC8468659
- DOI: 10.3390/ijms22189882
In Silico Study, Physicochemical, and In Vitro Lipase Inhibitory Activity of α, β-Amyrenone Inclusion Complexes with Cyclodextrins
Abstract
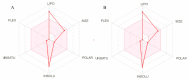
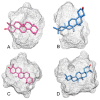
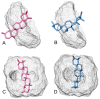
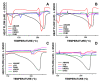
α,β-amyrenone (ABAME) is a triterpene derivative with many biological activities; however, its potential pharmacological use is hindered by its low solubility in water. In this context, the present work aimed to develop inclusion complexes (ICs) of ABAME with γ- and β-cyclodextrins (CD), which were systematically characterized through molecular modeling studies as well as FTIR, XRD, DSC, TGA, and SEM analyses. In vitro analyses of lipase activity were performed to evaluate possible anti-obesity properties. Molecular modeling studies indicated that the CD:ABAME ICs prepared at a 2:1 molar ratio would be more stable to the complexation process than those prepared at a 1:1 molar ratio. The physicochemical characterization showed strong evidence that corroborates with the in silico results, and the formation of ICs with CD was capable of inducing changes in ABAME physicochemical properties. ICs was shown to be a stronger inhibitor of lipase activity than Orlistat and to potentiate the inhibitory effects of ABAME on porcine pancreatic enzymes. In conclusion, a new pharmaceutical preparation with potentially improved physicochemical characteristics and inhibitory activity toward lipases was developed in this study, which could prove to be a promising ingredient for future formulations.
Keywords: amyrenone; cyclodextrins; inclusion complexes; lipase activity; triterpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ferreira R., Silva Júnior W., Veiga Junior V., Lima Á., Lima E., Ferreira R.G.S., Silva Júnior W.F., Veiga Junior V.F., Lima Á.A.N., Lima E.S. Physicochemical characterization and biological activities of the triterpenic mixture α,β-amyrenone. Molecules. 2017;22:298. doi: 10.3390/molecules22020298. - DOI - PMC - PubMed
-
- Pérez-González M.Z., Siordia-Reyes A.G., Damián-Nava P., Hernández-Ortega S., Macías-Rubalcava M.L., Jiménez-Arellanes M.A. Hepatoprotective and anti-inflammatory activities of the Cnidoscolus chayamansa (Mc Vaugh) leaf extract in chronic models. Evid. Based Complement. Alternat. Med. 2018;2018:3896517. doi: 10.1155/2018/3896517. - DOI - PMC - PubMed
-
- Santos F.A., Frota J.T., Arruda B.R., De Melo T.S., Da Silva A.A.D.C.A., Brito G.A.D.C., Chaves M.H., Rao V.S. Antihyperglycemic and hypolipidemic effects of α,β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012;11:98. doi: 10.1186/1476-511X-11-98. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

