Impact of Oncogenic Targets by Tumor-Suppressive miR-139-5p and miR-139-3p Regulation in Head and Neck Squamous Cell Carcinoma
- PMID: 34576110
- PMCID: PMC8469660
- DOI: 10.3390/ijms22189947
Impact of Oncogenic Targets by Tumor-Suppressive miR-139-5p and miR-139-3p Regulation in Head and Neck Squamous Cell Carcinoma
Abstract
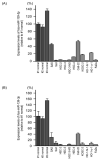
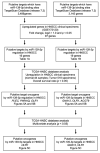
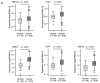
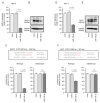
We newly generated an RNA-sequencing-based microRNA (miRNA) expression signature of head and neck squamous cell carcinoma (HNSCC). Analysis of the signature revealed that both strands of some miRNAs, including miR-139-5p (the guide strand) and miR-139-3p (the passenger strand) of miR-139, were downregulated in HNSCC tissues. Analysis of The Cancer Genome Atlas confirmed the low expression levels of miR-139 in HNSCC. Ectopic expression of these miRNAs attenuated the characteristics of cancer cell aggressiveness (e.g., cell proliferation, migration, and invasion). Our in silico analyses revealed a total of 28 putative targets regulated by pre-miR-139 (miR-139-5p and miR-139-3p) in HNSCC cells. Of these, the GNA12 (guanine nucleotide-binding protein subunit alpha-12) and OLR1 (oxidized low-density lipoprotein receptor 1) expression levels were identified as independent factors that predicted patient survival according to multivariate Cox regression analyses (p = 0.0018 and p = 0.0104, respectively). Direct regulation of GNA12 and OLR1 by miR-139-3p in HNSCC cells was confirmed through luciferase reporter assays. Moreover, overexpression of GNA12 and OLR1 was detected in clinical specimens of HNSCC through immunostaining. The involvement of miR-139-3p (the passenger strand) in the oncogenesis of HNSCC is a new concept in cancer biology. Our miRNA-based strategy will increase knowledge on the molecular pathogenesis of HNSCC.
Keywords: GNA12; HNSCC; OLR1; expression signature; miR-139-3p; miR-139-5p; microRNA; passenger strand; tumor suppressor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cohen E.E.W., Bell R.B., Bifulco C.B., Burtness B., Gillison M.L., Harrington K.J., Le Q.T., Lee N.Y., Leidner R., Lewis R.L., et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. Immunother. Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. - DOI - PMC - PubMed
-
- Wang X., Guo J., Yu P., Guo L., Mao X., Wang J., Miao S., Sun J. The roles of extracellular vesicles in the development, microenvironment, anticancer drug resistance, and therapy of head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2021;40:35. doi: 10.1186/s13046-021-01840-x. - DOI - PMC - PubMed
-
- Bonner J.A., Harari P.M., Giralt J., Cohen R.B., Jones C.U., Sur R.K., Raben D., Baselga J., Spencer S.A., Zhu J., et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

