Downregulated TNF-α Levels after Cryo-Thermal Therapy Drive Tregs Fragility to Promote Long-Term Antitumor Immunity
- PMID: 34576115
- PMCID: PMC8468796
- DOI: 10.3390/ijms22189951
Downregulated TNF-α Levels after Cryo-Thermal Therapy Drive Tregs Fragility to Promote Long-Term Antitumor Immunity
Abstract
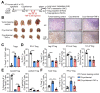
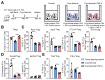
Immunotherapy has emerged as a therapeutic pillar in tumor treatment, but only a minority of patients get benefit. Overcoming the limitations of immunosuppressive environment is effective for immunotherapy. Moreover, host T cell activation and longevity within tumor are required for the long-term efficacy. In our previous study, a novel cryo-thermal therapy was developed to improve long-term survival in B16F10 melanoma and s.q. 4T1 breast cancer mouse models. We determined that cryo-thermal therapy induced Th1-dominant CD4+ T cell differentiation and the downregulation of Tregs in B16F10 model, contributing to tumor-specific and long-lasting immune protection. However, whether cryo-thermal therapy can affect the differentiation and function of T cells in a s.q. 4T1 model remains unknown. In this study, we also found that cryo-thermal therapy induced Th1-dominant differentiation of CD4+ T cells and the downregulation of effector Tregs. In particular, cryo-thermal therapy drove the fragility of Tregs and impaired their function. Furthermore, we discovered the downregulated level of serum tumor necrosis factor-α at the late stage after cryo-thermal therapy which played an important role in driving Treg fragility. Our findings revealed that cryo-thermal therapy could reprogram the suppressive environment and induce strong and durable antitumor immunity, which facilitate the development of combination strategies in immunotherapy.
Keywords: TNF-α; Treg fragility; cryo-thermal therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Boulch M., Cazaux M., Loe-Mie Y., Thibaut R., Corre B., Lemaître F., Grandjean C.L., Garcia Z., Bousso P. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci. Immunol. 2021;6:eabd4344. doi: 10.1126/sciimmunol.abd4344. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

