Celiac Disease Defined by Over-Sensitivity to Gliadin Activation and Superior Antigen Presentation of Dendritic Cells
- PMID: 34576145
- PMCID: PMC8469067
- DOI: 10.3390/ijms22189982
Celiac Disease Defined by Over-Sensitivity to Gliadin Activation and Superior Antigen Presentation of Dendritic Cells
Abstract
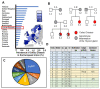
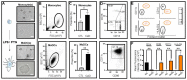
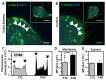
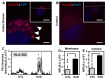
The autoimmune condition, Celiac Disease (CeD), displays broad clinical symptoms due to gluten exposure. Its genetic association with DQ variants in the human leukocyte antigen (HLA) system has been recognised. Monocyte-derived mature dendritic cells (MoDCs) present gluten peptides through HLA-DQ and co-stimulatory molecules to T lymphocytes, eliciting a cytokine-rich microenvironment. Having access to CeD associated families prevalent in the Czech Republic, this study utilised an in vitro model to investigate their differential monocyte profile. The higher monocyte yields isolated from PBMCs of CeD patients versus control individuals also reflected the greater proportion of dendritic cells derived from these sources following lipopolysaccharide (LPS)/ peptic-tryptic-gliadin (PTG) fragment stimulation. Cell surface markers of CeD monocytes and MoDCs were subsequently profiled. This foremost study identified a novel bio-profile characterised by elevated CD64 and reduced CD33 levels, unique to CD14++ monocytes of CeD patients. Normalisation to LPS stimulation revealed the increased sensitivity of CeD-MoDCs to PTG, as shown by CD86 and HLA-DQ flow cytometric readouts. Enhanced CD86 and HLA-DQ expression in CeD-MoDCs were revealed by confocal microscopy. Analysis highlighted their dominance at the CeD-MoDC membrane in comparison to controls, reflective of superior antigen presentation ability. In conclusion, this investigative study deciphered the monocytes and MoDCs of CeD patients with the identification of a novel bio-profile marker of potential diagnostic value for clinical interpretation. Herein, the characterisation of CD86 and HLA-DQ as activators to stimulants, along with robust membrane assembly reflective of efficient antigen presentation, offers CeD targeted therapeutic avenues worth further exploration.
Keywords: CD33; CD64; CD86; MHCDQ; autoimmunity; major histocompatibility complex II; monocyte; monocyte-derived dendritic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Enhanced Extracellular Transfer of HLA-DQ Activates CD3+ Lymphocytes towards Compromised Treg Induction in Celiac Disease.Int J Mol Sci. 2022 May 29;23(11):6102. doi: 10.3390/ijms23116102. Int J Mol Sci. 2022. PMID: 35682780 Free PMC article.
-
Circulating Dendritic Cells from Celiac Disease Patients Display a Gut-Homing Profile and are Differentially Modulated by Different Gliadin-Derived Peptides.Mol Nutr Food Res. 2020 Mar;64(6):e1900989. doi: 10.1002/mnfr.201900989. Epub 2020 Feb 5. Mol Nutr Food Res. 2020. PMID: 31970917
-
In vitro differentiation of human monocytes into dendritic cells by peptic-tryptic digest of gliadin is independent of genetic predisposition and the presence of celiac disease.J Clin Immunol. 2009 Jan;29(1):29-37. doi: 10.1007/s10875-008-9228-x. Epub 2008 Aug 12. J Clin Immunol. 2009. PMID: 18696220
-
[Celiac disease: clinical and subclinical forms].Allerg Immunol (Paris). 2002 Mar;34(3):100-2. Allerg Immunol (Paris). 2002. PMID: 12012789 Review. French.
-
Gluten Degrading Enzymes for Treatment of Celiac Disease.Nutrients. 2020 Jul 15;12(7):2095. doi: 10.3390/nu12072095. Nutrients. 2020. PMID: 32679754 Free PMC article. Review.
Cited by
-
The Immunological Epigenetic Landscape of the Human Life Trajectory.Biomedicines. 2022 Nov 11;10(11):2894. doi: 10.3390/biomedicines10112894. Biomedicines. 2022. PMID: 36428462 Free PMC article.
-
LRP-1 links post-translational modifications to efficient presentation of celiac disease-specific T cell antigens.Cell Chem Biol. 2023 Jan 19;30(1):55-68.e10. doi: 10.1016/j.chembiol.2022.12.002. Epub 2023 Jan 5. Cell Chem Biol. 2023. PMID: 36608691 Free PMC article.
-
Enhanced Extracellular Transfer of HLA-DQ Activates CD3+ Lymphocytes towards Compromised Treg Induction in Celiac Disease.Int J Mol Sci. 2022 May 29;23(11):6102. doi: 10.3390/ijms23116102. Int J Mol Sci. 2022. PMID: 35682780 Free PMC article.
-
Clarifying the Hazy Concepts of Food Allergies and Sensitivities.Gastroenterol Hepatol (N Y). 2024 Oct;20(9):524-531. Gastroenterol Hepatol (N Y). 2024. PMID: 39483998 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

