Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals-An Overview
- PMID: 34576159
- PMCID: PMC8469784
- DOI: 10.3390/ijms22189996
Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals-An Overview
Abstract
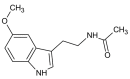
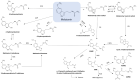
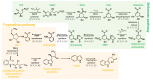
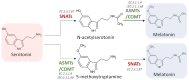
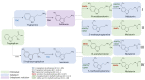
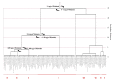
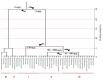
Melatonin is a ubiquitous indolamine, largely investigated for its key role in the regulation of several physiological processes in both animals and plants. In the last century, it was reported that this molecule may be produced in high concentrations by several species belonging to the plant kingdom and stored in specialized tissues. In this review, the main information related to the chemistry of melatonin and its metabolism has been summarized. Furthermore, the biosynthetic pathway characteristics of animal and plant cells have been compared, and the main differences between the two systems highlighted. Additionally, in order to investigate the distribution of this indolamine in the plant kingdom, distribution cluster analysis was performed using a database composed by 47 previously published articles reporting the content of melatonin in different plant families, species and tissues. Finally, the potential pharmacological and biostimulant benefits derived from the administration of exogenous melatonin on animals or plants via the intake of dietary supplements or the application of biostimulant formulation have been largely discussed.
Keywords: N-acetyl-5-methoxytriptamine; biostimulant; cluster analysis; dietary supplements; indolamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
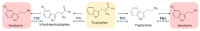
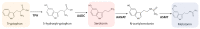



References
-
- Manchester L.C., Poeggeler B., Alvares F.L., Ogden G.B., Reiter R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995;41:391–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

